 Reaction Engg. & Thermodynamics Lab
Reaction Engg. & Thermodynamics Lab
To determine the rate constant for the saponification of ethyl acetate with sodium hydroxide in a plug flow reactor at ambient temperature. THEORY: In addition
 Experimental Studies of Ethyl Acetate Saponification Using Different
Experimental Studies of Ethyl Acetate Saponification Using Different
4 Feb 2019 ... reactor performance; pressure drop; volume flow ... Saponification of ethyl acetate using sodium hydroxide was considered to be a model reaction.
 CHEMICAL REACTION ENGINEERING LAB EXPERIMENT
CHEMICAL REACTION ENGINEERING LAB EXPERIMENT
We'll get back to this question later. 3) Determination of conversion in the tubular reactor•. Sodium hydroxide and ethyl acetate solutions are fed to the.
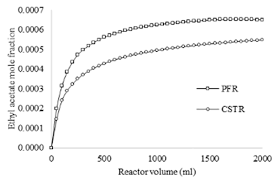
 A Comparative Study of Saponification Reaction in a PFR and CSTR
A Comparative Study of Saponification Reaction in a PFR and CSTR
6 Nov 2015 flow reactor (PFR). The reaction chosen for investigation was hydrolysis of ethyl acetate with sodium hydroxide. The objectives here are to ...
 CHEMICAL REACTION ENGINEERING LABORATORY LAB MANUAL
CHEMICAL REACTION ENGINEERING LABORATORY LAB MANUAL
for the saponification of ethyl acetate with NaOH. 2. To draw the performance chart for the reactor system and evaluate the reaction rate constant at ambient
 Experimental Investigations on Shifting Order Behavior of Ethyl
Experimental Investigations on Shifting Order Behavior of Ethyl
30 Jan 2020 As the past research reveals that the hydrolysis reaction of ethyl acetate under basic conditions is 2nd order but during experiments
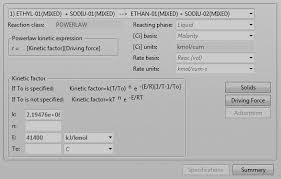
 MODELLING AND SIMULATION OF SAPONIFICATION REACTION
MODELLING AND SIMULATION OF SAPONIFICATION REACTION
This work presents the kinetic modelling and simulation of saponification of ethyl acetate in the presence of. Sodium hydroxide in a plug flow reactor
 Aspen Plus Simulation of Saponification of Ethyl Acetate in the
Aspen Plus Simulation of Saponification of Ethyl Acetate in the
Hydrolysis reaction of ethyl acetate with sodium hydroxide is in reaction kinetics o the saponification reaction and would be design of plug flow reactors.
 A Comparative Study of Alkaline Hydrolysis of Ethyl Acetate Using
A Comparative Study of Alkaline Hydrolysis of Ethyl Acetate Using
(0.01mol/L) and ethyl acetate (0.07mol/L) in batch and plug flow reactor. [24]) in literature regarding the saponification reaction of ethyl acetate ...
 Aspen Plus Simulation of Saponification of Ethyl Acetate in the
Aspen Plus Simulation of Saponification of Ethyl Acetate in the
sodium hydroxide in a plug flow reactor using Aspen Plus simulation software. Plug flow ... Hydrolysis reaction of ethyl acetate with sodium hydroxide.
 Experimental Studies of Ethyl Acetate Saponification Using Different
Experimental Studies of Ethyl Acetate Saponification Using Different
4 févr. 2019 performance of a chemical reactor because the reaction rate depends not only ... Saponification of ethyl acetate using sodium hydroxide was ...
 REACTION ENGINEERING 5.1. Determination of Kinetic Parameters
REACTION ENGINEERING 5.1. Determination of Kinetic Parameters
Sodium Hydroxide Mol wt.: 40.00 (pellet). 5.1.1. Aim. To study the saponification of ethyl acetate in a plug-flow reactor and to determine the kinetic.
 Eric T Henderson
Eric T Henderson
Another reactor design the plug flow reactor
 CHEMICAL REACTION ENGINEERING LAB EXPERIMENT
CHEMICAL REACTION ENGINEERING LAB EXPERIMENT
The NaOH concentration at the reactor outlet is once again measured by conductometry It is known that the ethyl acetate saponification reaction is.
 Effect of tubular reactor geometry on the saponification of ethyl
Effect of tubular reactor geometry on the saponification of ethyl
An aqueous solution of ethyl acetate and sodium hydroxide was al- lowed to react at amount calculated for a plug flow reactor and that for a stirred.
 Analysis of Chemical Reactors for Saponification Project: Final
Analysis of Chemical Reactors for Saponification Project: Final
In the laboratory the reaction of ethyl acetate and sodium hydroxide to form during testing
 A Comparative Study of Saponification Reaction in a PFR and CSTR
A Comparative Study of Saponification Reaction in a PFR and CSTR
6 nov. 2015 flow reactor (PFR). The reaction chosen for investigation was hydrolysis of ethyl acetate with sodium hydroxide. The.
 Experimental Study of the Influence of Process Conditions on
Experimental Study of the Influence of Process Conditions on
in maximizing the conversion of ethyl acetate saponification reaction for industrial Plug flow reactor (PFR); Conductivity; Conversion; Hydrolysis.
 EXPERIMENT 3 Saponification of Ethyl Acetate And Sodium
EXPERIMENT 3 Saponification of Ethyl Acetate And Sodium
The saponification process is a process that produces soap usually from fats and lye In technical terms saponification involves base (usually caustic soda NaOH) hydrolysis of triglycerides which are esters of fatty acids to form the sodium salt of a carboxylate
 Simulation and Optimization of Saponification of Ethyl Acetate in the
Simulation and Optimization of Saponification of Ethyl Acetate in the
The alkaline hydrolysis of acetate esters also know as the saponification reaction can be written as: AcO-R + Na+ + OH- ? AcO- + Na+ + R-OH (1) As the reaction proceeds hydroxide ions are consumed and acetate ions are produced
 Comparative Study of Saponification Reaction in a PFR and CSTR
Comparative Study of Saponification Reaction in a PFR and CSTR
Oil hydrolysis under antacid conditions is known as saponification5 Sodium acetate (CH3COONA) and ethanol (C2H5OH) are produced by hydrolysis of ethyl acetate by sodium hydroxide Numerous investigations were conducted on the advancement for this hydrolysis reaction
 MODELLING AND SIMULATION OF SAPONIFICATION REACTION IN
MODELLING AND SIMULATION OF SAPONIFICATION REACTION IN
This work presents the kinetic modelling and simulation of saponification of ethyl acetate in the presence of Sodium hydroxide in a plug flow reactor Continues stirred tank reactor using Aspen Plus simulation Software The continuous flow stirred-tank reactor (CSTR) also known as vat- or back mix reactor is a common ideal
 Optimization of Saponification Reaction in a Continuous
Optimization of Saponification Reaction in a Continuous
Saponification is the hydrolysis of ethyl acetate (EtOAc) to produce sodium acetate (CH 3 COONa) and ethyl alcohol (C 2 H 5 OH) using NaOH The stoichiometric representation of
 Estimation of Parameters of Arrhenius Equation for Ethyl
Estimation of Parameters of Arrhenius Equation for Ethyl
In this scientific research a Saponification Reaction between Ethyl Acetate and caustic soda is carried out in a Batch Reactor at STP Conditions The aim of this scientific research is to estimate the parameters of Arrhenius equation which are rate constant and activation energy for ethyl acetate saponification
Is saponification of ethyl acetate possible in a plug flow reactor?
- Subject This work presents the modelling and simulation of saponification of ethyl acetate in the presence of sodium hydroxide in a plug flow reactor using Aspen Plus simulation software. Plug flow reactors are widely used in the industry due to the non-mixing property.
What is the saponification reaction between sodium hydroxide (NaOH) and ethyl acetate (et?
- Introduction: In this reaction, the saponification reaction between sodium hydroxide (NaOH) and ethyl acetate (EtAc) was conducted. The reaction was conducted using equimolar amounts of both reactants to produce ethanol (EtOH) and sodium acetate (NaAc). In addition to, the kinetic parameters for the reaction were determined.
What is the simulation model for saponification of ethyl acetate?
- In this work, a simulation model was developed for the reaction of saponification of ethyl acetate in the presence of sodium hydroxide inside a plug flow reactor using Aspen Plus. The model was validated by the experimental results obtained from EDIBON Plug Flow Reactor module.
What chemicals were used in the saponification reaction?
- Chemicals: Analytical reagent (AR) - grade chemicals were utilized to conduct the experiments using PFR and CSTR. Ethyl acetate with 99.5% purity and sodium hydroxide with 98.0- 100% purity were used to carry out the saponification reaction.
[PDF] sara interregional guidelines for the evaluation of distance education
[PDF] sarf arabic pdf
[PDF] sars 2009 mexico
[PDF] sas 9.4 export to excel
[PDF] sas array
[PDF] sas array do loop
[PDF] sas array example ucla
[PDF] sas array find
[PDF] sas array init
[PDF] sas arrays
[PDF] sas arrays tutorial
[PDF] sas character array example
[PDF] sas create array from dataset
[PDF] sas enterprise guide 7.1 export to excel
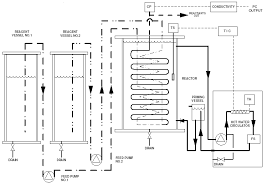 A Comparative Study of Alkaline Hydrolysis of Ethyl Acetate Using
A Comparative Study of Alkaline Hydrolysis of Ethyl Acetate Using