 Experiment 25: Calorimetry
Experiment 25: Calorimetry
CHEM 111 Morning Lab. 27 October 2014. Experiment 25: Calorimetry. Conclusion: The unknown metal #14 has a specific heat of 0.36 J/g °C; the heat of
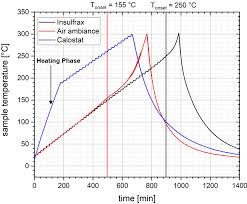 Experience Report on Adiabatic Reaction Calorimetry in Safety
Experience Report on Adiabatic Reaction Calorimetry in Safety
In conclusion the evaluation method has no significant influence on the In summary it has to pointed out that an interpretation of an adiabatic experiment ...
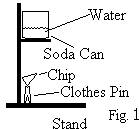
 Soda Can Calorimeter
Soda Can Calorimeter
In the 1770s Joseph Black (1728–1799) was one of the first scientists to conduct calorimetry experiments with different materials. He discovered that not
 Experiment 8 Calorimetry
Experiment 8 Calorimetry
This experiment will require most of the time allocated to the lab period. If you Report: The laboratory report should include Tables 1-4 (add titles to ...
 Calorimetry Lab Report On Planters Cashews Chemistry Period 3
Calorimetry Lab Report On Planters Cashews Chemistry Period 3
2 дек. 2015 г. Conclusion. The hypothesis created at the beginning the lab was incorrect. Throughout the attempts of finding the amount of calories in a ...
 AP CHEMISTRY SYLLABUS PHS 2017-2018
AP CHEMISTRY SYLLABUS PHS 2017-2018
Notebook Laboratory Reports- Always include the following in each report: Title The title should be descriptive. Experiment 5 is not a descriptive title. Date
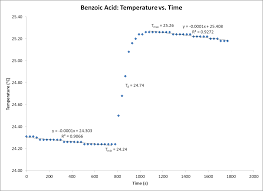
 Example Calorimetry Lab Report #2 – Good or In Need of Lots of
Example Calorimetry Lab Report #2 – Good or In Need of Lots of
26 окт. 2010 г. In this experiment the differences in caloric content of foods were investigated through the ignition of food items and the measurement of the ...
 Physical Chemistry Lab Report Rubric –Veldman Fall 2012
Physical Chemistry Lab Report Rubric –Veldman Fall 2012
We can conclude that the use of bomb calorimetry to determine the heat of combustion of sucrose was successful despite moderate experimental error. 3. Page 5
 Experiment 15: Specific Heat of a Metal
Experiment 15: Specific Heat of a Metal
Sep 5 2013 Conclusions: The specific heat of two metals was determined experimentally. The technique utilized was calorimetry. A sample of each metal was ...
 Writing a discussion
Writing a discussion
The discussion is the most important part of the laboratory report because it is here that you discussion for an introductory chemistry experiment.
 Example Calorimetry Lab Report #2 – Good or In Need of Lots of
Example Calorimetry Lab Report #2 – Good or In Need of Lots of
Oct 26 2010 In this experiment
 Experiment 25: Calorimetry
Experiment 25: Calorimetry
CHEM 111 Morning Lab. 27 October 2014. Experiment 25: Calorimetry. Conclusion: The unknown metal #14 has a specific heat of 0.36 J/g °C;
 Experiment 6 ? Calorimetry
Experiment 6 ? Calorimetry
Pre?lab questions Calorimetry is the science of measuring the quantities of heat ... customary to report its value on a per?mole basis. Thus we are.
 Finding the Specific Heat of a Substance
Finding the Specific Heat of a Substance
To measure specific heat in the laboratory a calorimeter of some kind must be In this experiment
 Coffee Cup Calorimetry
Coffee Cup Calorimetry
discussion of Pre-Lab Exercises regarding the difference in temperature is measured in calorimetry experiments. The ... and report its units.
 Bomb Calorimetry and Heat of Combustion
Bomb Calorimetry and Heat of Combustion
Nov 7 2014 In this experiment we used a Parr bomb calorimeter to accurately ... Raw data for this experiment is included in the Results section
 EXPERIMENT 12N CALORIMETRY
EXPERIMENT 12N CALORIMETRY
Aug 7 2018 Read the entire experiment and instructions

TEACHER PAGES
iCopyright © 2013 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
Chemistry
MATERIALS AND RESOURCES
EACH GROUP
balance data collection device graduated cylinder,100 mL
sensor, temperature, stainless steel weigh boat ammonium chloride calcium turningshydrochloric acid, 0.5 M sodium hydroxide, 0.5 M2 cups, 8-oz Styrofoam
with lids spoon, plastic water, distilledCoffee Cup
Calorimetry
Measuring Heat Transfer
ABOUT THIS LESSON
I n this lesson, students will evaluate a sample investigation to review concepts and techniques required for quantifying heat exchange in a high school laboratory. With some guidance, students will design calorimetry investigations for the physical process of an endothermic dissolution and two exothermic chemical reactions.OBJECTIVES
Students will:
• Review and evaluate sample procedures and data analysis for the heat exchange between metal and water in a coffee cup calorimeter • Design calorimetric investigations using parameters and assumptions for physical and chemical systems • Organize and analyze data to determine enthalpy changes for the processes carried out in their coffee cup calorimeters LEVELChemistry
TEACHER PAGES
iiCopyright © 2013 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
Chemistry - Coffee Cup Calorimetry
NEXT GENERATION SCIENCE STANDARDS
PLANNING/CARRYING OUT
INVESTIGATIONSANALYZING AND
INTERPRETING DATA
CONSTRUCTING EXPLANATIONS
DESIGNING SOLUTIONS
ENERGY AND MATTERSCALE, PROPORTION,
AND QUANTITY
PS3: ENERGY
ASSESSMENTS
The following types of formative assessments are
embedded in this lesson: • Assessment of student understanding through discussion of Pre-Lab Exercises regarding the sample investigation • Assessment of student-developed procedures and laboratory technique through active monitoring and questioning during investigationACKNOWLEDGEMENTS
Styrofoam
Brand is a registered trademark of The
Dow Chemical Company ("Dow") or an affiliated
company of Dow.TEACHER PAGES
iiiCopyright © 2013 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
Chemistry - Coffee Cup Calorimetry
COMMON CORE STATE STANDARDS
(LITERACY) RST.9-10.4Determine the meaning of symbols, key terms, and
other domain-specific words and phrases as they are used in a specific scientific or technical context relevant to grades 9-10 texts and topics. (LITERACY) W.1 Write arguments to support claims in an analysis of substantive topics or texts, using valid reasoning and relevant and sufficient evidence. (MATH) A-CED.4 Rearrange formulas to highlight a quantity of interest, using the same reasoning as in solving equations. (MATH) N-Q.1Use units as a way to understand problems and to
guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.CONNECTIONS TO AP*
5CHEMISTRYCHEMISTRY
APAP A.2The process of kinetic energy transfer
at the particulate scale is referred to in this course as heat transfer, and the spontaneous direction of the transfer is always from a hot to a cold body. B.1Energy is transferred between systems
either through heat transfer or through one system doing work on the other system. B.2When two systems are in contact with
each other and are otherwise isolated, the energy that comes out of one system is equal to the energy that goes into the other system. The combined energy of the two systems remains fixed. Energy transfer can occur through either heat exchange or work. B.3Chemical systems undergo three main
processes that change their energy; heating/cooling, phase transitions, and chemical reactions. B.4Calorimetry is an experimental technique
that is used to measure the change in energy of a chemical system. *Advanced Placement and AP are registered trademarks of the College Entrance Examination Board. The College Board was not involved in the production of this product.TEACHER PAGES
ivCopyright © 2013 National Math + Science Initiative, Dallas, Texas. All rights reserved. Visit us online at www.nms.org.
Chemistry - Coffee Cup Calorimetry
TEACHING SUGGESTIONS
Calorimetry
Prerequisite Knowledge and Skills
Understanding the relationship between mass, heat, and temperatureSolving for variables using the equation,
q = mCT (Eq. A) Differentiating between exothermic and endothermic processes Working with thermochemical equations to calculate heat or chemical amounts Relating heat transfer to the law of conservation of energyAccording to the law of conservation of energy,
0 = q gained + q lost (Eq. B) Manipulating the equation, the relationship between heat lost by one component of a system and the heat gained by another becomes q lost = q gained (Eq. C) This equation uses the same convention established in other lessons like "Mass, Heat, and Temperature" and "Burn, Baby, Burn." From the perspective of the system, the amount of heat gained (q gained ) is always a positive value. When values are substituted into the equation the directionality of heat lost (q lost ), always a negative value, is resolved and the magnitudes become equal. For the purposes of vertical alignment, check with the physics teachers to make sure that students are prepared for other variations of the heat variable. It is often q in chemistry but Q in physics. Students are trained that capitalization changes the variable entirely, such as with t (time) and T (temperature). If there is a difference between disciplines, make note of it with your students. Note that coffee cup calorimeters are not perfectly sealed and therefore maintain pressures equal to the barometric pressure in the room. The heat transfer, q, is equal to the change in enthalpy, H, for processes at constant pressure. Textbooks that introduce bomb calorimetry, referred to as constant-volume calorimetry, equate heat transfer to an overall change in energy, E. For the purposes of a first-year course, the approach to problem solving is the same for both types of calorimeters. The distinction between E and H, based on technical definitions for enthalpy, energy and work, is beyond the scope of the course. In the lab, students are asked to design their own procedures for three calorimetry investigations. A sample investigation - complete with procedures, data, and analysis - is presented to scaffold understandingquotesdbs_dbs2.pdfusesText_2[PDF] calweasel past exams
[PDF] cambridge curriculum for kindergarten
[PDF] cambridge curriculum guide
[PDF] cambridge curriculum online
[PDF] cambridge curriculum primary
[PDF] cambridge curriculum review
[PDF] cambridge curriculum schools in south africa
[PDF] cambridge curriculum south africa
[PDF] cambridge curriculum vs ib
[PDF] cambridge delta course locations
[PDF] cambridge english
[PDF] cambridge english course book
[PDF] cambridge english course book 9
[PDF] cambridge english course book pdf
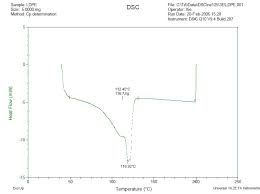 NE 125 - Experiment 3
NE 125 - Experiment 3