 Solutions
Solutions
are the molar masses of the solvent and solute respectively. From this equation (2.28) knowing all other quantities
 Coordination Compounds
Coordination Compounds
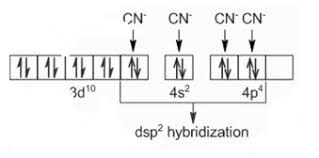
measurements in solution can be explained if (i) six groups in all either 1 Formulas of. Mononuclear. Coordination. Entities. 2022-23. Page 7. 250. Chemistry.
 NCERT Solutions for Class 12 Chemistry Chapter 9 Coordination
NCERT Solutions for Class 12 Chemistry Chapter 9 Coordination
Question 9.6(ii) Using IUPAC norms write the formulas for the following: (ii) Question 9.11(ii) Draw all the isomers (geometrical and optical) of: Answer ...
 Formulae For ELECTROCHEMISTRY
Formulae For ELECTROCHEMISTRY
12. κ. Λ m x1000. = C. Remember: Unit of Λm in above formula is Scm2mol-1. 13. α c m. 0 m. ∧. = ∧. 14. 2 a cα. K = 1-α. XII Chemistry. CHAPTER 3 -
 NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols
NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols
Q 11.3: (i) Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their. IUPAC names. (ii) Classify the isomers of alcohols in
 Biomolecules
Biomolecules
But all the compounds which fit into this formula may not be classified as 1 Class XII) are present in nucleic acids. Carbohydrates are found in ...
 CHEMISTRY (CLASSES XI –XII)
CHEMISTRY (CLASSES XI –XII)
CHEMISTRY (CLASSES XI –XII). RATIONALE. Higher Secondary Stage is the most solutions Henderson equation
 CHEMISTRY (Code No. 043) XI-XII (2023-24) Rationale Higher
CHEMISTRY (Code No. 043) XI-XII (2023-24) Rationale Higher
buffer solution Henderson Equation
 lech102.pdf
lech102.pdf
resistance of such a column of solution is then given by the equation: R = ρ l. A. = κ l. A. (2.17). Rationalised 2023-24. Page 14. 44. Chemistry. Table 2.3
 Class XII Chemistry Ch. 2: Solutions Important formulae & Concepts
Class XII Chemistry Ch. 2: Solutions Important formulae & Concepts
Number of parts of component. Parts per million. 10. Total number of parts of all components of solution. = ×. 5. Number of moles of solute. Molarity. Volumeof
 CBSE NCERT Solutions for Class 12 Chemistry Chapter 10
CBSE NCERT Solutions for Class 12 Chemistry Chapter 10
Give the structural formula of (a) and write the equations for all the reactions. Solution: There are two primary alkyl halides having the formula
 CBSE NCERT Solutions for Class 12 Chemistry Chapter 4
CBSE NCERT Solutions for Class 12 Chemistry Chapter 4
Now the rate equation will be: Rate2 = k(3a)2 = 9(ka2). Page 3. Class- XII-CBSE-Chemistry. Chemical Kinetics. Practice more on Chemical Kinetics. Page - 3 www.
 NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols
NCERT Solutions for Class 12 Chemistry Chapter 11 Alcohols
Q 11.3: (i) Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their. IUPAC names. (ii) Classify the isomers of alcohols in
 Solutions
Solutions
are the molar masses of the solvent and solute respectively. From this equation (2.28) knowing all other quantities
 CBSE NCERT Solutions for Class 12 Chemistry Chapter 11
CBSE NCERT Solutions for Class 12 Chemistry Chapter 11
Solution: (i). The structures of all isomeric alcohols of molecular formula C5H12O are shown below: Structural isomers
 NCERT Solutions (Updated for 2020-21 Academic Session) NCERT
NCERT Solutions (Updated for 2020-21 Academic Session) NCERT
Trigonometry Formulas. ? Integration Formulas Social Science – SST NCERT Solutions. ? Class 9 Social ... Chemistry MCQs for Class 12 Chapter Wise with.
 CBSE NCERT Solutions for Class 12 Chemistry Chapter 2
CBSE NCERT Solutions for Class 12 Chemistry Chapter 2
CBSE NCERT Solutions for Class 12 Chemistry Chapter 2 Formula required: Mass percentage of solute = ... Total number of moles of all components ...
 NCERT Solutions for Class 12 Chemistry Chapter 12 - Aldehydes
NCERT Solutions for Class 12 Chemistry Chapter 12 - Aldehydes
All the given reactions can be explained by the following equations. Question 12.12: Arrange the following compounds in increasing order of their property as.
 Chemical Kinetics - Formulas
Chemical Kinetics - Formulas
Chemical Kinetics - Formulas. All rates written as. ?conc. ?time or. ?[A]. ?t . Instantaneous rate is the slope of a concentration vs time plot and is.

Get the Power of Visual Impact on your side
Log on to
www.topperlearning.comClass XII
Chemistry
Ch. 2: Solutions
Important formulae & Concepts
1. Mass percentage of a component (w/w)
Mass of component in solution100Toal mass of solution× 2.Volume percentage of a component (v/v)
Volume of the component = 100Total volume of solution×3. Mole fraction of a component (x) =
Number of moles of the component
Total number of moles of all components
4.6Number of parts of componentParts per million10Total number of parts of all components of solution=×
5. Number of moles of soluteMolarityVolumeof solution in litres= 6. Number of moles of soluteMolalityMass of solvent in kilograms= 7. Number of gram equivalent of soluteNormalityVolumeof solution in litres= 8. o 1 1 2o 1 p pxp-= 9.Δ = -0
b bT T T b 2b2 1K 1000 wTM ×w× ×Δ =
10. 0 f fT T TΔ = - f 2f2 1K 1000 wTM ×w× ×Δ =
Get the Power of Visual Impact on your side
Log on to
www.topperlearning.com 11.π = CRT
12.22w RTM =Vπ
13.Normal molar massiAbnormal molar mass
Observedcolligative property
Calculatedcolligative property
Totalnumberofmolesofparticlesafterassoci
ation/dissociation on/dissociation14. Inclusion of van"t Hoff factor modified the equations for colligative
properties as: -=o 1 1 2 o11 n.n p pip× ×Δ =b 2b
2 1K 1000 wT .M ×wi
× ×Δ =f 2f
2 1K 1000 wT .M ×wi
=π2n RT.Vi15. According to Raoult"s law for a solution of volatile liquids the partial
vapour pressure of each component in the solution is directly proportional to its mole fraction. p1 = po1 x1 ; p2 = po2 x2
Using Dalton"s law of partial pressures the total pressure of solution is calculated. o o o total 1 2 1 2p =p +(p -p )xquotesdbs_dbs2.pdfusesText_2[PDF] all formulas of statistics class 11
[PDF] all formulas of statistics class 11 economics
[PDF] all formulas of statistics class 11 maths
[PDF] all formulas of statistics class 9
[PDF] all french verb tenses chart
[PDF] all google fonts list
[PDF] all guitar chords chart
[PDF] all guitar chords chart pdf
[PDF] all guitar chords for beginners
[PDF] all guitar chords pdf
[PDF] all guitar chords printable
[PDF] all guitar chords scales
[PDF] all guitar chords tabs
[PDF] all guitar chords with sound
