 Lab Session 9 Experiment 8: Calorimetry
Lab Session 9 Experiment 8: Calorimetry
https://www.ulm.edu/chemistry/courses/manuals/chem1009/session_09.pdf
 Experiment 6 ·∙ Calorimetry
Experiment 6 ·∙ Calorimetry
6-‐2a to compute. Ccal of your calorimeter. The molar enthalpy change of reaction ΔH. At constant pressure the heat in or out of a system is equal to.
 Physical Chemistry Lab Report Rubric –Veldman Fall 2012
Physical Chemistry Lab Report Rubric –Veldman Fall 2012
A Parr-type constant-volume bomb calorimeter was utilized for determination of the enthalpy of to successfully perform this bomb calorimetry lab.
 HEAT CAPACITY OF A CALORIMETER
HEAT CAPACITY OF A CALORIMETER
The total amount of heat that is produced or absorbed by a chemical reaction at constant pressure
 Experiment 6 ∙ Calorimetry
Experiment 6 ∙ Calorimetry
calorimeter constant Ccal of your calorimeter. What ... Experiment 6 ∙ Calorimetry. Lab report form. Page 3. (III.B) Report the quantities needed in the ...
 Chemistry 1009 Lab Manual University of Louisiana at Monroe
Chemistry 1009 Lab Manual University of Louisiana at Monroe
Lab Session 11 Experiment 10: Determination of the Molar Mass of Oxygen 8A Experiment: Determination of Calorimeter Constant. 1. Obtain or assemble a ...
 Enthalpy of Neutralization
Enthalpy of Neutralization
Calculations and Results. Calorimeter Constant Determination. Trial 1. 1. Mass of hot water (g). 2. Δt of hot water (oC)
 Experiment 5
Experiment 5
absorbed by the calorimeter can be determined and is referred to as the calorimeter constant. (Submit as part of your informal report). Part 1: Determining ...
 Enthalpies of Solution1 Authors: B. D. Lamp T. Humphry
Enthalpies of Solution1 Authors: B. D. Lamp T. Humphry
https://chemlab.truman.edu/files/2015/07/Enthalpies-of-Solution.pdf
 Enthalpies of Solution
Enthalpies of Solution
Nov 8 2015 Determination of the calorimeter constant. Calorimeter Setup. In this experiment
 Physical Chemistry Lab Report Rubric –Veldman Fall 2012
Physical Chemistry Lab Report Rubric –Veldman Fall 2012
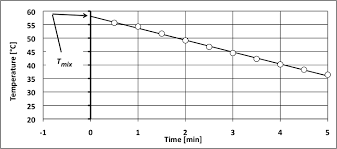
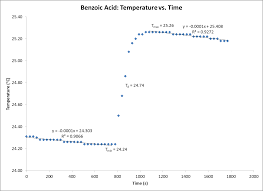
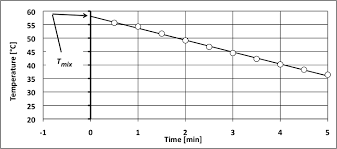
This value made possible the determination of the calorimeter constant. (Cvcal) which was calculated to be 8.78 kJ/?C. Figure 1 shows temperature vs. time for
 Lab Session 9 Experiment 8: Calorimetry
Lab Session 9 Experiment 8: Calorimetry
https://www.ulm.edu/chemistry/courses/manuals/chem1009/session_09.pdf
 Experiment 6 ·? Calorimetry
Experiment 6 ·? Calorimetry
(3) You will determine the calorimeter constant Ccal of your calorimeter. (I.B) Report the quantities needed for the calculation of Ccal according to ...
 Experiment 6 ? Calorimetry
Experiment 6 ? Calorimetry
(3) You will determine the calorimeter constant Ccal of your calorimeter. (I.B) Report the quantities needed for the calculation of Ccal according to ...
 Unit-3 Setting.pmd
Unit-3 Setting.pmd
(b) In the determination of calorimeter constant record the temperature of hot water just before mixing. (c) Avoid using very large amounts of copper sulphate/
 Experiment 12F CALORIMETRY AND HESSS LAW: FINDING ?H
Experiment 12F CALORIMETRY AND HESSS LAW: FINDING ?H
1 ???. 2010 ?. 2) Using equation (5) and your calorimeter constant C from Part A calculate ?Hb for the reaction involving Mg with HCl. Report your value in ...
 Chemistry 101 Experiment 7 - ENTHALPY OF REACTION USING
Chemistry 101 Experiment 7 - ENTHALPY OF REACTION USING
Chemistry 101 Experiment 7 - ENTHALPY OF REACTION USING HESS'S LAW Step 1 Determination of the Calorimeter Constant. ... Report Sheet. I. Determination ...
 HEAT CAPACITY OF A CALORIMETER
HEAT CAPACITY OF A CALORIMETER
Chem 1101 Lab. EXPERIMENT: CALORIMETRY AND HEAT OF NEUTRALIZATION. INTRODUCTION. Heat is defined as the transfer of energy from or into a system because of
 Enthalpies of Solution1 Authors: B. D. Lamp T. Humphry
Enthalpies of Solution1 Authors: B. D. Lamp T. Humphry
https://chemlab.truman.edu/files/2015/07/Enthalpies-of-Solution.pdf
[PDF] determine if function is linear calculator
[PDF] determine if function is linear exponential or neither
[PDF] determine if function is linear or nonlinear
[PDF] determine if two functions are linearly independent
[PDF] determine the fourier series coefficients for each of the following discrete time periodic signals
[PDF] determine the fourier series representation of the following periodic signal
[PDF] determine the fourier series representations
[PDF] determine the fourier series representations for the following signals
[PDF] determine the z transform
[PDF] determine the z transform for each of the following sequences
[PDF] determine the z transforms and sketch the roc of the following signals
[PDF] determine whether the functions are linearly dependant or independent
[PDF] déterminer l'ensemble des images d'une fonction
[PDF] déterminer l'ensemble des points m
