 SOLUTIONS & COLLIGATIVE PROPERTIES
SOLUTIONS & COLLIGATIVE PROPERTIES
This phenomenon is called reverse osmosis. Application : Desalination of sea water : When pressure more than osmotic pressure is applied pure water is squeezed
 Online Application of Colligative Properties Solutions Experiment
Online Application of Colligative Properties Solutions Experiment
Abstract. This study aimed to describe the practical implementation of colligative properties by Online. Method used in this study was pre-experiment.
 Colligative properties CH102 General Chemistry Spring 2014
Colligative properties CH102 General Chemistry Spring 2014
An important application of osmotic pressure is in the determination of molar masses of large molecules such as proteins. The reason is that usually
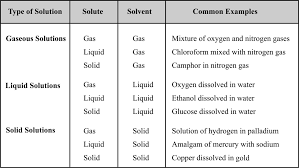
 Solutions
Solutions
Concentration described by mass percentage is commonly used in industrial chemical applications. colligative properties (colligative: from Latin: co means ...
 Multiple representation-based mobile apps with learning cycle 7e
Multiple representation-based mobile apps with learning cycle 7e
application of colligative properties of solutions that students have pdf. Page 12. “Multiple representation-based mobile apps with learning cycle 7e model ...
 REACT Strategy: Efforts to Link Concept Colligative Properties
REACT Strategy: Efforts to Link Concept Colligative Properties
Applying the concept of colligative properties of solutions is closely related to everyday life and science development so the REACT strategy can be applied in
 Diapositiva 1
Diapositiva 1
Definition of Colligative Property. • Vapour Pressure Lowering. • Freezing Point Depression (Cryoscopy). • Boiling Point Elevation (Ebullioscopy).
 How to develop colligative properties of solution chemistry e- book
How to develop colligative properties of solution chemistry e- book
Today school facilities have supported the implementation of digital-based learning. However
 International Journal of Instruction
International Journal of Instruction
5 Jun 2020 They were all not provided with chemical equipment and application of the Colligative Properties of. Solutions such as the sub-topic of ...
 Colligative Properties
Colligative Properties
colligative properties to measure the molecular weight of polymers. In applying boiling point elevation to polymer solutions we should realize that ...
 A Lecture on Colligative Properties in an Undergraduate Curriculum
A Lecture on Colligative Properties in an Undergraduate Curriculum
The effect of a solute on the vapor pressure may be determined in dilute solutions by applying the Raoult's Law (Eq. 1). o a. a a p = p x . (Eq.1).
 Colligative properties CH102 General Chemistry Spring 2014
Colligative properties CH102 General Chemistry Spring 2014
There are four colligative properties. • vapor-pressure lowering. • boiling-point elevation. • freezing-point depression. • osmotic pressure. Each of
 Formulas for Colligative Properties
Formulas for Colligative Properties
Formulas for Colligative Properties. Lowering of. Vapor Pressure. Elevating the. BOILING Point. Depression of the. FREEZING Point. Osmotic. Pressure.
 COLLIGATIVE-PROPERTIES.pdf
COLLIGATIVE-PROPERTIES.pdf
Application of Colligative properties (i) Explain the term Colligative property. (ii) State four Colligative properties of solution.
 Untitled
Untitled
Colligative Properties of. Electrolytes. •. Solution Dosage Forms. •. Application of Colligative. Properties. II. Colligative Properties of. Solutions.
 Developing Innovative Chemistry Laboratory Workbook Integrated
Developing Innovative Chemistry Laboratory Workbook Integrated
of Colligative Properties of solutions. The implementation of InoChemLaW was carried out onto the experimental class compared to the existing laboratory.
 Colligative Properties of Foods
Colligative Properties of Foods
Colligative Properties. 3.1. Depression of the Freezing Point. 3.1.1. Basic Concepts. 3.1.2 Applications to Foods. 3.2. Elevation of the Boiling Point.
 WORKSHEET:SOLUTIONS AND COLLIGATIVE PROPERTIES SET
WORKSHEET:SOLUTIONS AND COLLIGATIVE PROPERTIES SET
WORKSHEET:SOLUTIONS AND COLLIGATIVE PROPERTIES. SET A: 1. Find the molarity of all ions in a solution that contains 0.165 moles of aluminum chloride in 820.
 Lecture 4: Colligative Properties
Lecture 4: Colligative Properties
By definition a colligative property is a solution property (a property of mixtures) for which it is the amount of solute dissolved in the solvent matters

Chapter 5
Colligative Properties
5.1 Introduction
Properties of solutions that depend on the number of molecules present and not on the kind ofmolecules are called colligative properties. These properties include boiling point elevation, freezing
point depression, and osmotic pressure. Historically, colligative properties have been one means for determining the molecular weight of unknown compounds. In this chapter we discuss using colligative properties to measure the molecular weight of polymers. Because colligative properties depend on the number of molecules, we expect, and will show, that colligative property experiments give a number average molecular weight.5.2 Boiling Point Elevation
Figure 5.1 shows the vapor pressure of a liquid for pure liquid and for a solution with that liquid as
the solvent. In an ideal solution, the vapor pressure of the solvent,PA, is reduced from the vaporpressure of a pure liquid,P◦A, toXAP◦AwhereXAis the mole fraction of liquidA. This reduction is
reflected in a shift to the right of the vapor-pressure curves in Fig. 5.1. By definition, boiling point
is the temperature at which the vapor pressure of the liquid reaches 1 atm. Thus, the right-shift caused by the dissolution of componentBin solventAcauses the boiling point to increase. This increase, ΔTb, is the boiling point elevation effect. A well known result from introductory chemistry is that the boiling point elevation is propor- tional to the molar concentration of solute particlesΔTb=Kbm(5.1)
where m is the molality of solute molecules andKbis the boiling point elevation coefficient that is a function of only the solvent. Molality is the number of moles of componentBper 1000 grams of solvent. If we prepare a solution of an unknown compound of molecular weightBat a concentration 6566CHAPTER 5. COLLIGATIVE PROPERTIESVapor PressureVapor Pressure
SolutionTemperaturePure Solvent
1 atm TbDTFigure 5.1: Boiling point elevation effect is a consequence of the effect of solute molecules on the vapor
pressure of the solvent. cin g/cm3, then m=1000cMBρ(5.2) whereρis the density of the solvent (in g/cm3). Substituting into the expression for ΔTbgives MB=1000KbcρΔTb(5.3)
orΔTbc=1000KbρMB(5.4)
For a given solvent (e.g., water whereKb= 0.52 andρ= 1.00) and concentration (c), all terms in Eq. (5.4) are known except forMB. Thus, measuring ΔTbcan be used to determine the molecular weightMB. We can also express boiling point elevation in terms of mole fraction. Mole fraction is XB=cVMBρVMA+cVMB≈cMAρMB(5.5)
whereVis total volume andMAis molecular weight of the solvent. The boiling point elevation becomesΔTb=1000KbMAXB(5.6)
To apply boiling point elevation to polymers, we begin by using solution thermodynamics to derive an expression for ΔTb. At equilibrium, the chemical potential of the vapor is equal to the chemical potential of the liquid vapA=μliq
A=μ◦A+RTlnXAorμvap
A-μ◦ART= lnXA(5.7)
5.2. BOILING POINT ELEVATION67
where we have assumed an ideal solution. Differentiating both sides gives ?μAT?∂T=∂?GAT?∂T=1T?
∂GA∂T? P -GAT2=-SAT-HAT2+SAT=-HAT2(5.8) which is used to get HvapA-H◦ART2=ddTlnXA(5.9)
whereHvap A-H◦Ais the heat of vaporization of the solvent or ΔHvap. Now consider the process of forming a solution. As the polymer is added, the mole fraction of Awill go from 1 at the start toXAwhich is the mole fraction of the final solution. During the process, the boiling point will go fromTbtoTwhereTbis the boiling point of the pure liquid and Tis the boiling point of the solution. Integrating over this process gives T T bΔHvapRT2dT=? XA1dlnXA(5.10)
The integrals are easily evaluated if we assume that ΔHvapis independent of temperature over the small temperature range fromTbtoT. The result isΔHvapR?
1T-1Tb?
= lnXA(5.11) We can simplify this result using ΔTb=T-Tb,TTb≈T2b, and lnXA= ln(1-XB)≈ -XB. These simplifications apply whenXBis small (which occurs when the solution is dilute) and when ΔTbis small. In general, ΔTbwill be small when the solution is dilute. The previous equation simplifies toΔHvapΔTbRT2b=XB(5.12) orΔTb=RT2bΔHvapXB(5.13)
Comparison of this result to Eq. (5.6) gives a theoretical expression ofKb: K b=MART2b1000ΔHvap(5.14) The result is often derived in physical chemistry books. In applying boiling point elevation to polymer solutions, we should realize that polymer solu- tions are really solutions of many components. The various components are the polymer species of different molecular weights. Because boiling point elevation is a colligative property, we can write the boiling point elevation of a polymer solution as a sum over the mole fractions of each molecular weight component:ΔHvapΔTbRT2b=? iX i(5.15)68CHAPTER 5. COLLIGATIVE PROPERTIES
whereXiis the mole fraction of polymer with molecular weightMi. We more conveniently rewrite X iin terms of concentration: X i=c iVMiρVMA+? ic iVMi≈ciMAρMi(5.16) whereciis the concentration in weight/unit volume (e.g., g/cm3) of polymer with molecular weight i. The approximation in this expression is valid for dilute solutions in which the number of moles of solvent is much greater than the total number of moles of polymer. Summing the mole fractions, X i, results in? iX i=cMAρ? ic iMi? ici=cMAρ? iw iMi=cMAρMN(5.17) where c=? ic i(5.18) The final expression for the boiling point elevation becomesΔTbc=MART2bρΔHvapMN(5.19)
It is common to express the boiling point elevation in terms of the latent heat of vaporization,lvap,
defined as energy or vaporization per unit weight or l vap=ΔHvapMA=J/moleg/mole= heat of vaporization in J/g (5.20)The boiling point elevation becomes
ΔTbc=RT2bρlvapMN(5.21)
Except for incorporation of polydispersity, there is nothing new about the boiling point eleva- tion expression for polymer solutionsvs.the comparable expression for small molecule solutions. In polymers, however, the solution is more likely to be non-ideal. For this equation to apply we will probably need to use very low concentrations or techniques to extrapolate to very low concen- trations. For an example, let"s consider a solution of polystyrene in benzene. For benzeneρ= 0.8787 g/cm3, T b= 55◦C, andlvap= 104 cal/g. We assume a relatively concentrated solution ofc= 1 g/cm3 of a polymer with molecular weightMN= 20,000. The change in the boiling point elevation forthis solution is ΔTb= 1.4×10-3◦C. This boiling point elevation is very small. It is probably
beyond the accuracy of most temperature measuring equipment. The small change arises despite relatively ideal conditions of a fairly concentrated solution and a low molecular weight polymer. More dilute solutions or higher molecular weight polymers would give an even smaller ΔTb. The problem with polymer solutions is that for a given weight of material, the polymer solution will5.3. FREEZING POINT DEPRESSION69
have many less molecules than the comparable small molecule solution. When there are a small number of molecules, the change in boiling point (a colligative property) is small. The problem with the boiling point elevation method applied to polymer solutions is that it is not sensitive enough. It has found some use with polymers but it is limited to polymers with relatively low molecular weights. (e.g.,MNless than 20,000 g/mol).5.3 Freezing Point Depression
A similar analysis (but with sign changes) can be applied to the freezing point depression of a polymer solution. The final result isΔTfc=RT2fρlfMN(5.22)
whereTfis the freezing point of the solvent andlfis the latent heat of fusion. We consider the same example of polystyrene in benzene withTf= 5.5◦C,lf= 30.45 cal/g for the freezing point of benzene. For ac= 1 g/cm3solution of polystyrene with molecular weightMN= 20,000, thechange in the freezing point of the solution is ΔTf= 2.9×10-3◦C. Like the boiling point elevation
effect, the freezing point depression effect is too small. The technique is insensitive and only useful
for low molecular weight polymer (e.g.,MNless than 20,000 g/mol).5.4 Osmotic Pressure
Another colligative property is osmotic pressure. Figure 5.2 illustrates the osmotic pressure ef- fect. Imagine a pure solvent and a solution separated by a semipermeable membrane. An ideal semipermeable membrane will allow the solvent molecules to pass but prevent the solute molecules (polymer molecules) from passing. The different concentrations on the two sides of the membrane will cause an initial difference in chemical potential. At equilibrium, this difference in potential will be counteracted by an effective pressure across the membrane. As shown in Fig. 5.2, it can be imagined that solvent molecules pass from the pure solvent side to the solution side. The excessheight in the column of liquid above the solution side is related to the osmotic pressure byπ=ρgh.
Hereπis the osmotic pressure,ρis the density of the solution,gis the acceleration of gravity (9.81 m/sec2) andhis the height of the column of liquid.
We begin with a thermodynamic analysis of osmotic pressure. At equilibrium the chemical potential in the solution will be equal to the chemical potential in the pure solvent: whereμsolventAis the chemical potential of the pure liquid or solventA=μ◦A(5.24)70CHAPTER 5. COLLIGATIVE PROPERTIESSolventSolutionMembrane
hp = rghFigure 5.2: A schematic view of osmotic pressure across a semipermeable membrane. The only way the chemical potentials will be equal will be if the activity of componentAin thequotesdbs_dbs2.pdfusesText_3[PDF] application of derivatives in daily life pdf
[PDF] application of derivatives in physics pdf
[PDF] application of derivatives pdf
[PDF] application of derivatives pdf target
[PDF] application of derivatives ppt
[PDF] application of derivatives problems with answers pdf
[PDF] application of e learning in education
[PDF] application of fermentation
[PDF] application of fermentation in food industry
[PDF] application of fir filter in medical
[PDF] application of gps
[PDF] application of laplace transform
[PDF] application of laplace transform to boundary value problems
[PDF] application of laplace transform to differential equations calculator
