 Reaction synthesis and kinetic modeling of isoamyl acetate via
Reaction synthesis and kinetic modeling of isoamyl acetate via
Results show that there is two main reactions took place which are: (i) between acetic anhydride and isoamyl alcohol
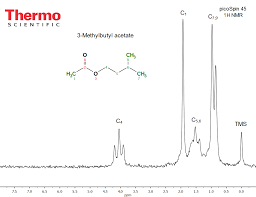 Lesson Plan: Synthesis of Isopentyl Acetate (Banana Oil)
Lesson Plan: Synthesis of Isopentyl Acetate (Banana Oil)
isopentyl acetate (3-methylbutyl acetate) via an esterification reaction between acetic acid and isopentyl alcohol (3-methylbutanol) using concentrated ...
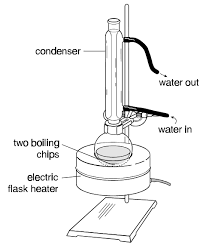 Synthesis of Isopentyl Acetate
Synthesis of Isopentyl Acetate
Synthesis of Isopentyl Acetate. 8. Objectives. To prepare isopentyl acetate from isopentyl alcohol and acetic acid by the Fischer esterification reaction.
 Thermodynamic and Kinetic Study on the Catalysis of Isoamyl
Thermodynamic and Kinetic Study on the Catalysis of Isoamyl
Sep 29 2020 equilibrium constants of acetic acid
 Parameters study on the production of isoamyl acetate via milli
Parameters study on the production of isoamyl acetate via milli
Non-catalyzed reaction was performed by reacting isoamyl alcohol with acetic anhydride without further dilution. The flow rate of 40 to 80µL/min reaction.
 Experiment 14A: Isopentyl Acetate
Experiment 14A: Isopentyl Acetate
Oct 14 2020 A Fischer esterification is an acid-catalyzed reaction in which a carboxylic acid and an alcohol react to form an ester and water.1 In this ...
 Nomination Background: Isoamyl acetate (CASRN: 123-92-2)
Nomination Background: Isoamyl acetate (CASRN: 123-92-2)
It can also be prepared by esterification of commercial isoamyl alcohol with acetic acid (Opdyke 1975). mechanism of drunkenness. The ester administration ...
 ARTIGO ORIGINAL ESTERIFICATION OF ACETIC ACID WITH
ARTIGO ORIGINAL ESTERIFICATION OF ACETIC ACID WITH
For the system with mechanical agitation the variables studied were molar ratio acetic acid and isoamyl alcohol (1:4.18-1:9.82) and reaction temperature (35.9-
 isoamyl acetate 1.0.5.pages
isoamyl acetate 1.0.5.pages
The reaction of an alcohol with a carboxylic acid is a straightforward way to synthesize esters. Often these esters have an interesting aroma.
 reaction synthesis and kinetic modeling of isoamyl acetate via
reaction synthesis and kinetic modeling of isoamyl acetate via
reaction of isoamyl acetate in solvent-free system (SFS)
 Lesson Plan: Synthesis of Isopentyl Acetate (Banana Oil)
Lesson Plan: Synthesis of Isopentyl Acetate (Banana Oil)
via an esterification reaction between acetic acid and isopentyl alcohol (3-methylbutanol) using concentrated sulfuric acid as a catalyst.
 Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by
Producing Natural Flavours from Isoamyl Alcohol and Fusel Oil by
10 jui. 2022 In cyclohexane the best performer reaction solvent
 Green synthesis of banana flavor using different catalysts: a
Green synthesis of banana flavor using different catalysts: a
25 fév. 2020 Esterification of isoamyl alcohol with acetic acid was studied using ... and isoamyl alcohol (mole ratio of 1:2) after 2 h of reaction time.
 Parameters study on the production of isoamyl acetate via milli
Parameters study on the production of isoamyl acetate via milli
Non-catalyzed reaction was performed by reacting isoamyl alcohol with acetic anhydride without further dilution. The flow rate of 40 to 80µL/min reaction.
 Kinetics and mechanism of esterification of isoamyl alcohol with
Kinetics and mechanism of esterification of isoamyl alcohol with
In this study the effects of the rate enhancement of isoamyl acetate formation by immobilized Mucor miehei lipase catalysed esterification of isoamyl
 Synthesis of Isopentyl Acetate.pdf
Synthesis of Isopentyl Acetate.pdf
To prepare isopentyl acetate from isopentyl alcohol and acetic acid by the Fischer esterification reaction. Introduction. Esters are derivatives of carboxylic
 Analysis of water content in esterification of isoamyl acetate by using
Analysis of water content in esterification of isoamyl acetate by using
an analytical tool to detect water content in esterification reaction between isoamyl alcohol and acetic anhydride. The effects of split ratio and carrier
 Thermodynamic and Kinetic Study on the Catalysis of Isoamyl
Thermodynamic and Kinetic Study on the Catalysis of Isoamyl
29 sept. 2020 acetic acid to isoamyl alcohol temperature
 Preparation of Isoamyl Acetate by High Performance ZSM-5 Zeolite
Preparation of Isoamyl Acetate by High Performance ZSM-5 Zeolite
When the reaction temperature acetic acid/isoamyl alcohol molar ratio
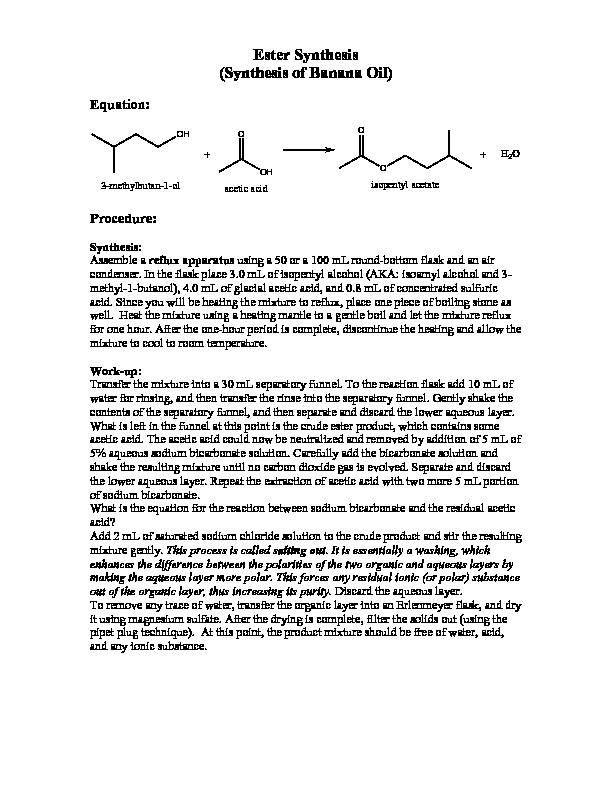
Ester Synthesis
(Synthesis of Banana Oil)Equation:
3-methylbutan-1-ol
acetic acid isopentyl acetate +H2OProcedure:
Synthesis:
Assemble a reflux apparatus using a 50 or a 100 mL round-bottom flask and an air condenser. In the flask place 3.0 mL of isopentyl alcohol (AKA: isoamyl alcohol and 3- methyl-1-butanol), 4.0 mL of glacial acetic acid, and 0.8 mL of concentrated sulfuric acid. Since you will be heating the mixture to reflux, place one piece of boiling stone as well. Heat the mixture using a heating mantle to a gentle boil and let the mixture reflux for one hour. After the one-hour period is complete, discontinue the heating and allow the mixture to cool to room temperature.Work-up:
Transfer the mixture into a 30 mL separatory funnel. To the reaction flask add 10 mL of water for rinsing, and then transfer the rinse into the separatory funnel. Gently shake the contents of the separatory funnel, and then separate and discard the lower aqueous layer. What is left in the funnel at this point is the crude ester product, which contains some acetic acid. The acetic acid could now be neutralized and removed by addition of 5 mL of5% aqueous sodium bicarbonate solution. Carefully add the bicarbonate solution and
shake the resulting mixture until no carbon dioxide gas is evolved. Separate and discard the lower aqueous layer. Repeat the extraction of acetic acid with two more 5 mL portion of sodium bicarbonate. What is the equation for the reaction between sodium bicarbonate and the residual acetic acid? Add 2 mL of saturated sodium chloride solution to the crude product and stir the resulting mixture gently. This process is called salting out. It is essentially a washing, which enhances the difference between the polarities of the two organic and aqueous layers by making the aqueous layer more polar. This forces any residual ionic (or polar) substance out of the organic layer, thus increasing its purity. Discard the aqueous layer. To remove any trace of water, transfer the organic layer into an Erlenmeyer flask, and dry it using magnesium sulfate. After the drying is complete, filter the solids out (using the pipet plug technique). At this point, the product mixture should be free of water, acid, and any ionic substance.Final Purification by Distillation:
Assemble a simple distillation apparatus using clean and dry glassware. Choose the distillation flask based on the volume of the partly purified banana oil you have. Normally, the volume of the mixture should be between one half-to-two thirds of the volume of the flask. Start heating and distilling. The product of interest (isopentyl acetate) will be collected between 134 and 143 oC. Monitor the temperature closely. You may collect the distillates at lower temperatures in a graduated cylinder, but you should collect the product of interest in a clean and dry pear-shaped flask with a side arm.For your report:
Weigh the product (the distillate) and determine the % Yield. Obtain an IR spectrum of the product and compare it to the literature IR given to you by your instructor.quotesdbs_dbs2.pdfusesText_4[PDF] acetic acid and salicylic acid reaction
[PDF] acetic acid anhydride synthesis
[PDF] acetic acid as a catalyst for the n acylation of amines using esters as the acyl source
[PDF] acetic acid bacteria anaerobic
[PDF] acetic acid bacteria benefits
[PDF] acetic acid bacteria fermentation
[PDF] acetic acid bacteria in kombucha
[PDF] acetic acid bacteria in sourdough
[PDF] acetic acid bacteria vinegar
[PDF] acetic acid conversion to acetic anhydride
[PDF] acetic acid fermentation
[PDF] acetic acid fermentation ppt
[PDF] acetic acid fermentation process
[PDF] acetic acid flammable
