 UNIT- II: Aromatic Acids - Acidity effect of substituents on acidity and
UNIT- II: Aromatic Acids - Acidity effect of substituents on acidity and
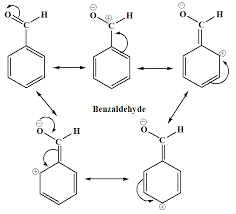
➢ Why Benzoic acid will not undergo Friedel-Craft Reaction? - Because -COOH group present in aromatic carboxylic acids is an electron withdrawing group causing.
 Chapter 3 Alcohols Phenols
Chapter 3 Alcohols Phenols
https://www.angelo.edu/faculty/kboudrea/index_2353/Chapter_03_2SPP.pdf
 ORGANIC CH B. Sc. II YEAR ORGANIC CHEMISTRY HEMISTRY-II
ORGANIC CH B. Sc. II YEAR ORGANIC CHEMISTRY HEMISTRY-II
6.3 Nomenclature of carboxylic acids. 6.4 Structure and bonding. 6.5. Physical properties. 6.6 Acidity of carboxylic acids and effect of substituents on acid
 PRACTICAL LAB MANUAL
PRACTICAL LAB MANUAL
The general formula for an aliphatic carboxylic acid is RCOOH and for an aromatic carboxylic acid is ArCOOH. mixture until it becomes acidic ad benzoic acid ...
 SAMPLE PREPARATION FUNDAMENTALS FOR
SAMPLE PREPARATION FUNDAMENTALS FOR
recovered by elution with a non-polar acidic solvent such as hexane/EtOAc with 1% acetic acid. carboxylic acid (RCOOH) from the donor side by first ...
 Nomenclature of Carboxylic Acids
Nomenclature of Carboxylic Acids
Esters may be broken apart under acidic conditions by water (a hydrolysis reaction) to form a carboxylic acid and an alcohol. • This is essentially the reverse
 Synthesis and Chemistry of Indole
Synthesis and Chemistry of Indole
Step 3: Cyclization to indole‐2‐carboxylic acid. Step 4: Decarboxylation. Page 4. By Dr. Divya Kushwaha. 2.4 Bartoli Indole Synthesis: ➢ Efficient and
 chemistry/xii-(2020-21)
chemistry/xii-(2020-21)
Carboxylic Acids: Nomenclature acidic nature
 PCI Syllabus: B.Pharm UNIT –V: Carboxylic Acids The carboxyl
PCI Syllabus: B.Pharm UNIT –V: Carboxylic Acids The carboxyl
to silver mirror and Fehling's solution to red ppt. and itself gets Since conjugation of carboxylic acid to aryl rings is known to increase the its acidity.
 Chapter 1 Organic Compounds: Alkanes Organic chemistry
Chapter 1 Organic Compounds: Alkanes Organic chemistry
– Many functional groups contain oxygen atoms such as alcohols
 CARBOXYLIC ACIDS
CARBOXYLIC ACIDS
Substituents mainly exert their influence on the acidity of aliphatic carboxylic acid through the inductive effect. Since the inductive effect operates through
 UNIT- II: Aromatic Acids - Acidity effect of substituents on acidity and
UNIT- II: Aromatic Acids - Acidity effect of substituents on acidity and
There are several categories of aromatic acids including: (i) Phenolic acids: substances containing an aromatic ring and an organic carboxylic acid function
 Carbonyl Chemistry (12 Lectures) Aldehydes and Ketones
Carbonyl Chemistry (12 Lectures) Aldehydes and Ketones
The carbonyl group may be further oxidized to carboxylic acids Addition of water to carbonyl compounds under acidic conditions is analogous.
 14: Substituent Effects
14: Substituent Effects
Chapter 14. 14: Substituent Effects. Substituents and Their Effects. Carboxylic Acid Acidity. SN1 Reactions. Electrophilic Aromatic Substitution Reactions.
 Mitsunobu Reaction
Mitsunobu Reaction
carboxylic acids in the presence of diethyl azodicarboxylate. (DEAD) [A0705] and triphenylphosphine (TPP) Furthermore the p?a of the usable acidic.
 Chapter 3 Alcohols Phenols
Chapter 3 Alcohols Phenols
https://www.angelo.edu/faculty/kboudrea/index_2353/Chapter_03_2SPP.pdf
 Lab Manual - Pharmaceutical Organic Chemistry
Lab Manual - Pharmaceutical Organic Chemistry
Functional group test: test for carboxylic acid 3- Aq. solution + CaCl2: A white ppt. of Ca oxalate is separated ... 2- It gives Acidity test +ve.
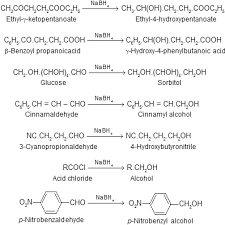
 Chapter 21: Carboxylic Acid Derivatives
Chapter 21: Carboxylic Acid Derivatives
all derivatives of carboxylic acids: carboxylic acid ester anhydride acyl halides amides ... basic or acidic conditions the latter being more common.
 AMINO ACIDS CLASSIFICATION AND PROPERTIES
AMINO ACIDS CLASSIFICATION AND PROPERTIES
Acidic amino acids: have carboxyl group in their side chain. Eg: Aspartic and Glutamic acid. • Basic amino acids: contain amino group in their side chain.
 Synthesis and Chemistry of Indole
Synthesis and Chemistry of Indole
Step 3: Cyclization to indole?2?carboxylic acid Reaction involves acidic treatment of 2-arylamino-ketones (produced from a 2–halo-ketone.
[PDF] acidity of carboxylic acids with halogens
[PDF] acidity of drinking water
[PDF] acidity of phenol
[PDF] acidity of water
[PDF] acidity order of carboxylic acid derivatives
[PDF] acip certification
[PDF] acknowledgement for seminar report in engineering pdf
[PDF] acls and bls course in symbiosis 2020
[PDF] acls apply
[PDF] acls fellowship application
[PDF] acls grant application
[PDF] acls instructor
[PDF] acls instructor class
[PDF] acls instructor classes near me
