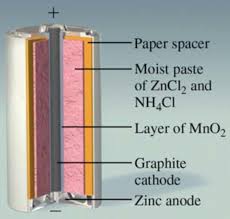
 Chapter 18: Electrochemistry
Chapter 18: Electrochemistry
Standard cell potential : The cell potential under standard state conditions Once the half-cell potentials are determined we can calculate E°cell. E cell.
 (}:: ~ ~ ~ ~u~ cJ
(}:: ~ ~ ~ ~u~ cJ
co.J.I~
 Untitled
Untitled
Calculate the standard cell potential E° cell
 Untitled
Untitled
Peb 14 2003 Given the following data
 Exercise 18.8 - Non-Standard Potentials & Electrolysis - Answers
Exercise 18.8 - Non-Standard Potentials & Electrolysis - Answers
Calculate Ecell at 25°C for this battery when [H2SO4] = 4.5 M that is [H+] = [HSO4'] = 4
 Exercise 18.2 - Standard Cell Potentials - Answers
Exercise 18.2 - Standard Cell Potentials - Answers
DIRECTIONS: Write the oxidation and reduction half reactions. Calculate the standard cell potential for the following electrochemical cells. 3. Ag+(aq) + Fe(s)
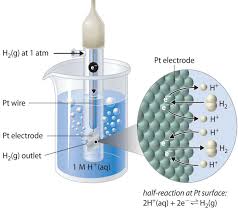 Cell Potential
Cell Potential
Mar 18 2020 at 298 K. We write the equation for the spontaneous reaction and calculate. E° cell from standard electrode potentials then ...
 Study Guide for Electrochemistry How to find K from E°cell 1. To review
Study Guide for Electrochemistry How to find K from E°cell 1. To review
o E°cell is the standard cell potential (in V) o R is the thermodynamic form Calculate the solubility product constant for Ag2MoO4(s). Solution: • We know ...
 Electrochemical Cells Worksheet
Electrochemical Cells Worksheet
Calculate the standard cell potential produced by a galvanic cell consisting of a nickel electrode in contact with a solution of Ni2+ ions and a silver
 T H E U N I V E R S I T Y O F S Y D N E Y
T H E U N I V E R S I T Y O F S Y D N E Y
The relevant half cell reactions and reduction potentials are: Cu2+(aq) + 2e- → Cu calculate the cell potential. The reaction involves the movement of 2 ...
 Ch.14-16 Electrochemistry Redox Reaction - the basics
Ch.14-16 Electrochemistry Redox Reaction - the basics
E°red of zero. • The potential of a cell can be calculated from standard reduction potentials: (. ) (. ) anode cathode red red cell. °?.
 Cell Potential
Cell Potential
18 mars 2020 at 298 K. We write the equation for the spontaneous reaction and calculate. E° cell from standard electrode potentials then ...
 Pre-Lab Questions/Answers – Experiment 6
Pre-Lab Questions/Answers – Experiment 6
Calculate the standard fuel cell potential Eo (or open circuit potential) from their standard half- cell reduction potentials. 4. For per mole of CH3OH
 CHEM1612 2014-N-12 November 2014 • In the electrolytic
CHEM1612 2014-N-12 November 2014 • In the electrolytic
12 nov. 2014 Using the relevant electrode potentials found on the data page calculate the reduction potential at 298 K of a half-cell formed by: (a) an Ag ...
 Untitled
Untitled
Given that the standard reduction potential for Cr2O72?2C³ is 1.33 V what is E°Red for I2(aq)? 10) Calculate E°cell and AG for the following reaction:.
 CHEM1101 2014-J-14 June 2014 • An electrochemical cell consists
CHEM1101 2014-J-14 June 2014 • An electrochemical cell consists
14 juin 2014 Calculate the standard cell potential E°cell
 Exercise 7 POTENTIOMETRIC MEASUREMENT OF pH
Exercise 7 POTENTIOMETRIC MEASUREMENT OF pH
Electrode potentials are often expressed and calculated by the Nernst equation derived from thermodynamics of the electrochemical cell.
 Chapter 4 Theoretical Calculation of Reduction Potentials
Chapter 4 Theoretical Calculation of Reduction Potentials
There are several approaches to calculating a condensed-phase reduction potential ranging from phenomenological or theoretically guided linear free energy
 Untitled
Untitled
Calculating Standard Cell Potentials E cell. 5. The standard cell potential for a pair of electrodes is sum of the reduction potential and oxidation
 Chapter 18: Electrochemistry
Chapter 18: Electrochemistry
Standard cell potential : The cell potential under standard state Once the half-cell potentials are determined we can calculate E°cell. E cell.
 [PDF] Cell Potential
[PDF] Cell Potential
18 mar 2020 · Any potential we measure will be its cathodic or reduction potential 2020-03-18 Page 19 Determining an unknown E°
 [PDF] Chapter 18: Electrochemistry
[PDF] Chapter 18: Electrochemistry
Measured current o called the overall cell potential (Ecell) o is the difference between the electrical potentials at the two electrodes (the two half-cell
 [PDF] Chapter 18
[PDF] Chapter 18
Calculations of cell potentials and equilibrium constants for redox reactions as well as for redox titration curves can sometimes differ significantly from
 236: Calculating Standard Cell Potentials - Chemistry LibreTexts
236: Calculating Standard Cell Potentials - Chemistry LibreTexts
9 août 2022 · The table below can be used to determine the reactions that will occur and the standard cell potential for any combination of two half-cells
 [PDF] Electrode Potentials
[PDF] Electrode Potentials
Although we cannot determine absolute potentials of electrodes we can easily determine relative electrode potentials Substituting other electrodes while
 [PDF] Topic 9 Electrochemistry
[PDF] Topic 9 Electrochemistry
23 jan 2018 · But there is no way to measure the potentials of individual electrode processes Arbitrarily divide total cell potential to assign potentials
 [PDF] Nernst Equation
[PDF] Nernst Equation
The Nernst equation is often used to calculate the cell potential of an electrochemical cell at any given temperature pressure and reactant concentration
 [PDF] Topic 111 Electrode Potentials and Cells - AQA Chemistry A-level
[PDF] Topic 111 Electrode Potentials and Cells - AQA Chemistry A-level
Standard cell potential values are used to calculate the overall cell EMF This is always done as potential of the right of the cell minus the potential of
 [PDF] Electrochemical Cells Worksheet
[PDF] Electrochemical Cells Worksheet
Electrochemical Cells Worksheet 1 Calculate the standard cell potential produced by a galvanic cell consisting of a nickel electrode in contact
- The standard potential, E°, for a redox reaction is related to the standard change in free energy, ?G°, for the reaction by the equation ?G° = -nFE°.
[PDF] how to calculate credit rating of a company
[PDF] how to calculate currency exchange
[PDF] how to calculate density of water at different temperatures
[PDF] how to calculate dilution factor
[PDF] how to calculate dilution factor for cell counting
[PDF] how to calculate dilution factor from concentration
[PDF] how to calculate effective address in 8086
[PDF] how to calculate epinephrine dose
[PDF] how to calculate exchange rate
[PDF] how to calculate experimental yield
[PDF] how to calculate february days
[PDF] how to calculate floor area
[PDF] how to calculate freight cost
[PDF] how to calculate frequency spectrum
