SUBSTITUTION REACTIONS OF METAL
SUBSTITUTION REACTIONS OF METAL
2 F. Basolo and R. G. Pearson Mechanisms of Inorganic Reactions
 INORGANIC CHEMISTRY RADIOCHEMISTRY
INORGANIC CHEMISTRY RADIOCHEMISTRY
reaction mechanism. [See p. 166 in Basolo and. Pearson (I 7). ] ligands may alter the reaction path. For example the rates of hydrolysis of a series of cis ...
 Chemical Kinetics and Inorganic Reaction Mechanisms - Second
Chemical Kinetics and Inorganic Reaction Mechanisms - Second
Basolo. R. E. Connick
 Summer School Karlsruhe-Dublin
Summer School Karlsruhe-Dublin
reaction.4. References. [1] F. Basolo R.G. Pearson
 Untitled
Untitled
3. Mechanisms of Inorganic Reactions; 2nd edn. ; F. Basolo R.G. Pearson; Wiley; 1967. 4. Reaction Mechanism of Inorganic and Organometallic
 Raman spectra of polyborate ions in aqueous solution
Raman spectra of polyborate ions in aqueous solution
Basolo and R. G. Pearson Mechanisms o[ Inorganic. Reactions
 Éloge: Fred Basolo (1920–2007)
Éloge: Fred Basolo (1920–2007)
20-Jan-2008 Pearson. Their classic book Mechanisms of Inorganic Reactions [10]
 SUBSTITUTION REACTIONS OF METAL COMPLEXES
SUBSTITUTION REACTIONS OF METAL COMPLEXES
2 F. Basolo and R. G. Pearson Mechanisms of Inorganic Reactions
 Course No: CHM-201 - Title: Inorganic Chemistry
Course No: CHM-201 - Title: Inorganic Chemistry
Mechanisms of Inorganic Reactions 2nd ed. - F. Basolo
 INORGANIC CHEMISTRY RADIOCHEMISTRY
INORGANIC CHEMISTRY RADIOCHEMISTRY
MECHANISMS OF SUBSTITUTION REACTIONS. OF METAL COMPLEXES. Fred Basolo and Ralph G . Pearson. Deportment of Chemistry. Northwestern University. Evanrton.
 Mechanisms of Inorganic Reactions : The Role of Ion Association in
Mechanisms of Inorganic Reactions : The Role of Ion Association in
These observations were interpreted in terms of a dual mechanism but were later challenged successfully by Basolo Henry
 Untitled
Untitled
Unit-I Reaction Mechanism in Coordination Complexes I (16 Contact hours) Mechanisms of Inorganic Reactions; 2nd edn. ; F. Basolo R.G. Pearson; Wiley; ...
 Chemical Kinetics and Inorganic Reaction Mechanisms - Second
Chemical Kinetics and Inorganic Reaction Mechanisms - Second
Basolo. R. E. Connick
 Mechanisms of inorganic reactions-A study of metal complexes in
Mechanisms of inorganic reactions-A study of metal complexes in
determine reaction order and then how one might postdate a mechanism. exclusively from inorganic chemistry. ... Fred Basolo and Ralph G. Pewson
 Ligand Substitution
Ligand Substitution
02-May-2006 Basolo and R. G. Pearson Mechanisms of Inorganic Reactions
 Inorganic Reaction Mechanisms
Inorganic Reaction Mechanisms
The Basolo and Pearson's book Mechanisms of Inorganic. Reactions (1958) probably marks the beginning of a systematic mechanistic approach to.
 SUBSTITUTION REACTIONS OF METAL
SUBSTITUTION REACTIONS OF METAL
continued interest in the mechanisms of reactions of metal complexes. 2 F. Basolo and R. G. Pearson Mechanisms of Inorganic Reactions
 Mechanism of the Acid Hydrolysis of Pentammine Cobalt Complex
Mechanism of the Acid Hydrolysis of Pentammine Cobalt Complex
mechanism for the acid hydrolysis reaction (l) has not as yet
 Mechanisms of inorganic reactions; a study of metal complexes in
Mechanisms of inorganic reactions; a study of metal complexes in
4 sept 2019 · Mechanisms of inorganic reactions; a study of metal complexes in solution by: Basolo Fred 1920- Associated-names: Pearson Ralph G
 [PDF] Mechanisms of Inorganic Reactions Fred Basolo Ralph G Pearson
[PDF] Mechanisms of Inorganic Reactions Fred Basolo Ralph G Pearson
Mechanisms of inorganic reactions in solution an introduction Denis Benson Hard and soft acids and bases Ralph G Pearson 1973 Science 480 pages
 F Basolo and RG Pearson Mechanisms of Inorganic Reactions
F Basolo and RG Pearson Mechanisms of Inorganic Reactions
F Basolo and R G Pearson Mechanisms of Inorganic Reactions: A Study of Metal Complexes in Solution 2nd Edn HOME · F Basolo and R G Pearson
 [PDF] Inorganic Reaction Mechanism - Utkal University
[PDF] Inorganic Reaction Mechanism - Utkal University
Fred Basolo (1920 – 2007) Henry Taube (1915-2005) Nobel Prize - 1983 Ralph Pearson (1919 - ) Experimental work in Inorganic reaction mechanisms
 Mechanisms of inorganic reactions - A study of metal complexes in
Mechanisms of inorganic reactions - A study of metal complexes in
Inevitably the hydrogen evolution reaction is very extensively covered but many other reactions are mentioned Examples are drawn almost exclusively from
 Mechanisms of Inorganic Reactions : The Role of Ion Association in
Mechanisms of Inorganic Reactions : The Role of Ion Association in
In this paper we will discuss the substitution reactions of complexes of the type (4) Basolo F Pearson R "Mechanisms of Inorganic Reactions" p
 Mechanisms of inorganic reactions a study of metal complexes in
Mechanisms of inorganic reactions a study of metal complexes in
Mechanisms of inorganic reactions a study of metal complexes in solution Authors: Fred Basolo Ralph G Pearson (Author) Front cover image for Mechanisms
 [PDF] INORGANIC CHEMISTRY RADIOCHEMISTRY
[PDF] INORGANIC CHEMISTRY RADIOCHEMISTRY
research activity in these very important areas of physical-inorganic chemistry Basolo F and Pearson R G “Mechanisms of Inorganic Reactions
 Mechanisms of Substitution Reactions of Metal Complexes
Mechanisms of Substitution Reactions of Metal Complexes
View in Scopus Basolo and Pearson 1958 F Basolo R G Pearson “Mechanisms of Inorganic Reactions ” Wiley New York (1958)
What are the 4 types of general inorganic reactions?
Almost every inorganic chemical reaction falls into one or more of four broad categories. ? Behaviour in presence acid, base, metal ions, nucleophiles, electrophiles, solvents alone or in combination with variation of reaction parameters.How do you memorize reactions in Inorganic chemistry?
The five basic types of chemical reactions are combination, decomposition, single-replacement, double-replacement, and combustion.
Inorganic Reactions Mechanism
Nigamananda Das
Department of Chemistry
Utkal University, Bhubaneswar
Outline
Introduction
Inorganic Reactions : Types
Classification/Mechanisms
Substitution Reactions of complexes
Conclusions
offourbroadcategories.CombinationReactions(addition)
S(s)+O2(g)՜SO2(g)
DecompositionReactions
2HgO(s)+heat(energy)՜2Hg(l)+O2(g)
Zn(s)+CuSO4(aq)՜Cu(s)+ZnSO4(aq)
Solid state Reaction, Photochemical
General Types of Inorganic Reactions
Reactions of Metal Complexes
[Co(NH 3)5(O 2CCO2H)]Cl
2Substitution
RedoxCoordinated
ligandIsomerisation
Addition
[Pt(NH3)4]2++ Cl-ĺ[Pt(NH3)3Cl]++ NH3 [Ru(NH3)6]3++ [Cr(H2O)6]2+ĺ [Ru(NH3)6]2++ Cr(H2O)6]3+ [Cr(H2O)6]3++ OH-ĺ[Cr(H2O)5(OH)]2++ H2OCu(acac)2+ pyĺCu(acac)2py
(CN=5) (CN=5)Reactions & Mechanisms of Complexes
Reactionmechanisminvolves"..
pathways molecularlevel.Rate :For a general reaction
A + B M + N
Rate = -d[A]/dt= -d[B]/dt= d[M]/dt= d[N]/dt
Rate Law: The rate law is the experimentally determined dependence of the reaction rate on reagent concentrationRate = k [A] [B]
determinedexperimentallyfromkineticstudy Early Theoretical Work in Inorganic Reaction MechanismsVan Vleck/Hartmann
Crystal-field Stabilization Energy
M. Dewar
Perturbational molecular
orbital theoryR. Hoffmann
Extended Hückel theory
Reactions & Mechanisms of Complexes
Fred Basolo
(1920 Ȃ2007)Henry Taube (1915-2005)
Nobel Prize -1983
Ralph Pearson
(1919 -) Experimental work in Inorganic reaction mechanisms started after World War II (~ 1945 )Alfred Werner (1866 1919): A
Swiss chemist won the Nobel Prize
in Chemistry in 1913 for proposing the basis of modern Coord. Chem.Sophus Mads Jørgensen(1837-
1914): A Danish chemist. Considered
founder of Coordination Chemistry.Manfred Eigen (1927 -)
(Nobel Prize 1967, Fast reaction)Reactions & Mechanisms of Complexes
Classification of reactions of metal complexes
Substitution
Electrophilic:Replacement of one metal by another
MLnΪ ǯ MLn-1ǯ Ϊ
Nucleophilic:Replacement of one ligand by another
Co(NH3)5X-+ H2O Co(NH3)5OH22++ X-
Co(NH3)5Cl2++ OH-Co(NH3)5OH2++ Cl-
Co(NH3)5H2O3++ L-Co(NH3)5L2++ H2O
ML4+ Y ՜ML3Y + L
Oxidation-reduction
Inner-sphere : (NH3)5CoCl + Cr(OH2)6-Ϊ ՜ Co(OH2)62++ 5NH4++ Cr(OH2)5Cl2+ Outer-sphere : FeII(CN)64-/ FeIII(CN)63+; Cr(OH2)62-/ Co(en)33+Sterochemicalchanges
Cis-trans Isomerisation
Reaction of Coordinated ligands
Dissociative(D) (SN1 lim) (Essentially same as SN1 in Organic Chemistry) An intermediate of lower CN than the reactant can be identified; rarely isolatable. The bond between the metal and the leaving group has been completely broken in the T.S. without any bond makingMechanisms of ligand exchange reactions:
Nucleophilic substitution
MLnX MLnY
MLnXYDissociative (D)
Two step mechanism:
Formation of intermediate: ML5X ML5+ X (Slow)
Attack of incoming group: ML5+ Y ML5Y (Fast)
ML L L L LLML L L LTBPSq. Py
Kinetics of Dissociative reactions: Rate Law
Using Steady State Approximation: Conc. of intermediates are small and constant over most of the course of a reactionAssociative (A) (SN2 lim) :
An intermediate of higher CN than the reactant can be identifiedMLnX MLnY
MLnXYYX
Associative (A)
Two step mechanism:
Formation of intermediate: ML5X + Y ML5XY (Slow; Int. rarely isolatable) Release of leaving group : ML5XY ML5Y + X (Fast)Kinetics of Associative
reactionsMechanisms of ligand exchange reactions:
Nucleophilic substitution
LL L LXM Y L M L LL Y X L LCapped Oct.Penta bipy
ǻve
For short lived
Intermediate or
TSInterchange(I):
Idmechanism:
The reaction rate is more sensitive to changes in the leaving group Large degree of bond breaking to the leaving group in T.S. Small amount of bond making to the entering group in T. S.ML5X + Y ֖
(O.S.) (k)T.S. (fast)Iamechanism:
The reaction rate is more sensitive to changes in the entering groupSome bond breaking to the leaving group in T.S.
Large degree of bond making to the entering group in T. S.ML5X + Y ֖
(O.S.)(k)T.S. (fast)MLnX MLnY
[MLn]°YX XYMechanisms of ligand exchange reactions:
Nucleophilic substitution
Kinetics of interchange reactions
Fast equilibrium
K1= k1/k-1
k2<< k-1For [Y] >> [ML5X]
SubstitutionMechanism
D (SN1 lim)IdIaA (SN2 lim)
Evidence of
intermediate with reduced CNNo definite
evidence --No definite evidenceEvidence of
intermediate with increased CNBond breaking is
the rdsGreaterinfluence
of bond breaking in rdsGreaterinfluence
of bond making in rdsBond making is
the rdsOverall classification
Experimental evidence for dissociative mechanisms
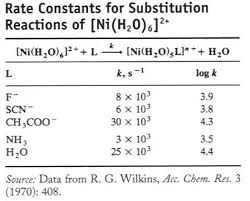
Rate is independent of the nature of L
Factors affecting M-X bond
strengthCharge (decrease)/Size (increase)
on metal centreCharge (decrease)/Size (increase)
of leaving groupCharge (increase)/Size (increase)
on other ligands (spectator).Incoming ligand identity (L) has no
effect on rate. for octahedral substitutions because one molecule splits into two at the intermediate (care should be taken for solvation effect). "Less common in substitution reaction of octahedral complexes "Identity of entering ligand (L) influences the rate coming together (Associative) "Negative ¨Vvalues because two species combine into one, with a presumed volume smaller than the total for the reactantsExperimental evidence for associative mechanisms
andlargeratevariation indicateassociationas partoftheTS(lamechanism).ForRu(II)ratesare
nearlythesamefor differentligands(Idmechanism)Substitution Reaction of Square planar complexes
complexeswithstrongligandsCo+Ni2+Cu3+
Rh+Pd2+Ag3+
Ir+Pt2+Au3+
Cl Cl -2
PtCl Cl
Cl Cl -1
PtCl NH3
Cl NH3Pt
Cl NH3
H3N Cl
PtCl NH3
Cl NH3Pt
Cl NH3
H3N Cl
PtCl NH3[ ][ ]
+NH3 -Cl- -NH3 +Cl- trans cis trans cisH3N NH3 +2
PtH3N NH3
H3N NH3 +
PtH3N Cl
Kinetics and stereochemistry
L MLL X L MLL Y L MLL X L MLL X L MLL S L MLL S S Y Y +Y +S -X +Y -S -X k2, slow k1, slowYinrds(solventassistedpath).
inrds.Thisstepfasterthanthesolventpath. constantthroughoutthereaction. tbptransitionstate.ML3ĺ3Y + X
Rate = -d[ML3X]/dt = kobs[ML3X]
= k1[ML3X] + k2[Y][ML3X]Rate = kobs[ML3X] = k1[ML3X] + k2[Y][ML3X]
Plotofkobsversus[Y],straightlinewith
k1=intercept&k2=slopewhichdepends onthenucleophilicitystrengthofY.¾Forallincomingligand,thek1values
(fromintercept)aremoreorlessconstant indicatingtheygothroughthesame specieswhilek2valuesaredifferentfrom oneligandtoanother.Nucleophile Y, entering
group from above or below the plane, coordinates to give a tbp intermediate which loses X with retention of stereochemistry.Kinetics and stereochemistry
(stericeffect),Evidences in favour of Associative mechansim
2.Formationof5-coordinatedintermediate
andhavespaceforYtocoordinate.3.Verificationofratelaw:
canverifytheratelawEvidences in favour of Associative mechansim
Role of the entering group
Role of the leaving group
Effect of metal centre
Nature of the other ligands in the complex
1.RoleoftheEnteringGroup
nucleophilicityofenteringgroupi.e.for mostreactnofPt(II),therateconst. increasesintheorder:H2O~Cl- PR3>AsR3>SbR3>>NR3
Sulphur>Oxygen
Hencesoftbasesarebetternucleophiles
forPt(II))asitisasoftacidmetalcentre. Factors affecting the rate of substitution
ɄPt = logk2(Y)/k1(CH3OH)
2.Roleofleavinggroup
For [Pt(dien)X]++ py [Pt(dien)(py)]++ X-in H2O at 25oC The sequence of lability is: H2O > Cl->Br-> I-> N3-> SCN-> NO2- > CN-with a spread of over 106in rate across series. The series tend to parallel the strength of the Metal-L bond. 3. Effect of the Metal Centre
The order of reactivity of a series of isovalent ions is: Ni(II) > Pd(II) >> Pt(II) which is same as that of the tendency to form 5-coordinate complexes. For M(II) = kNi(33 M-1s-1) > kPd(0.58 M-1s-1) > kPd (6.7x10-6M-1s-1). Factors affecting the rate of substitution
4. The Nature of other Ligands in the Complex
(a)Trans-effect (b)Steric effect Factors affecting the rate of substitution
The Trans Effect
introducedforthefirsttime. T M LX LT M LY L +X, -Y Order of trans
effect Pt(II) complexes Synthetic applications of the trans effect
toPt(II)complexes. Explanations for the Trans Effect
transitionstate:PtX3T(T=transgroup) (a)Poortranseffect,lowgroundstate. calledtransinfluence) Forstrongı-donors(H-,PR3,I-,Me-etc.)
thePtpxanddx2-y2orbitals andleaveslessforthePt-Xbond. breakingofthisbond. length)andNMR(reduced1JM-Lvalues) H-> PR3> SCN-> I-~ CH3-~ CO ~ CN-> Br-> Cl-> NH3> OH- Explanations for the Trans Effect
propertiesoftheligands. orbitaloftheseȺ- Explanations for the Trans Effect
combinationofthetwoeffects abilities times Explanations for the Trans Effect
Theories of trans-effect
1. Polarization Theory (Grinberg1935)
polarizingligandwillbethetrans-director. Pt(II)cationinducesadipoleinthe
polarizabletrans-directingligand.The induceddipoleinligandAinducesadipolein releaseofCl-duetoextrarepulsiveforce. 2. Ⱥ-Bonding Theory (stabilization of T.S.) : Orgel& Chatt(1956)
Hydrolysis:Substitution reactninvolving replacement of ligand by H2O/OH- Acid Hydrolysis (Aquation): Product is an aqua complex ML5Xn++ H2O ML5(H2O)n+1+ X-
Base Hydrolysis: Product is a hydroxocomplex
ML5Xn++ OH-ML5(OH)n+ X-
Anation(Replacement of coordinated water): Reverse of acid hydrolysis ML5(OH2)n++ X-ML5Xn-1+ H2O
Water Exchange reactions
Solovolysis
Types of octahedral substitution reactions
canbeputintofourclasses. Ɇe.g. alkalaimetals and larger alkaline earths
-Ǣ γ ͳ-5to 108sec-1
Ɇe.g. dipositive transition metals and tri-positive lanthanides 2- Ǣ γ ͳ - ͳ-4sec-1 Ɇe.g. most of the tri-positive transition metals, Be+2and Al+3 3 ȋ-... "-ȌǢ γ ͳ--1to 10-9sec-1
e.g. Cr+3(d3); Co+3(LS d6); Pt+2(LS d8); Rh+2; Ru+2 causecrystalfieldtobecomelessstable. Kinetics of Octahedral Substitution
Rate measurements
ForInertSystems:
Direct chemical analysis : argentomentry
Spectrophotometric methods
quotesdbs_dbs17.pdfusesText_23
PR3>AsR3>SbR3>>NR3
Sulphur>Oxygen
Hencesoftbasesarebetternucleophiles
forPt(II))asitisasoftacidmetalcentre.Factors affecting the rate of substitution
ɄPt = logk2(Y)/k1(CH3OH)
2.Roleofleavinggroup
For [Pt(dien)X]++ py [Pt(dien)(py)]++ X-in H2O at 25oC The sequence of lability is: H2O > Cl->Br-> I-> N3-> SCN-> NO2- > CN-with a spread of over 106in rate across series. The series tend to parallel the strength of the Metal-L bond.3. Effect of the Metal Centre
The order of reactivity of a series of isovalent ions is: Ni(II) > Pd(II) >> Pt(II) which is same as that of the tendency to form 5-coordinate complexes. For M(II) = kNi(33 M-1s-1) > kPd(0.58 M-1s-1) > kPd (6.7x10-6M-1s-1).Factors affecting the rate of substitution
4. The Nature of other Ligands in the Complex
(a)Trans-effect (b)Steric effectFactors affecting the rate of substitution
The Trans Effect
introducedforthefirsttime. T M LX LT M LY L +X, -YOrder of trans
effect Pt(II) complexesSynthetic applications of the trans effect
toPt(II)complexes.Explanations for the Trans Effect
transitionstate:PtX3T(T=transgroup) (a)Poortranseffect,lowgroundstate. calledtransinfluence)Forstrongı-donors(H-,PR3,I-,Me-etc.)
thePtpxanddx2-y2orbitals andleaveslessforthePt-Xbond. breakingofthisbond. length)andNMR(reduced1JM-Lvalues) H-> PR3> SCN-> I-~ CH3-~ CO ~ CN-> Br-> Cl-> NH3> OH-Explanations for the Trans Effect
propertiesoftheligands. orbitaloftheseȺ-Explanations for the Trans Effect
combinationofthetwoeffects abilities timesExplanations for the Trans Effect
Theories of trans-effect
1. Polarization Theory (Grinberg1935)
polarizingligandwillbethetrans-director.Pt(II)cationinducesadipoleinthe
polarizabletrans-directingligand.The induceddipoleinligandAinducesadipolein releaseofCl-duetoextrarepulsiveforce.2. Ⱥ-Bonding Theory (stabilization of T.S.) : Orgel& Chatt(1956)
Hydrolysis:Substitution reactninvolving replacement of ligand by H2O/OH- Acid Hydrolysis (Aquation): Product is an aqua complexML5Xn++ H2O ML5(H2O)n+1+ X-
Base Hydrolysis: Product is a hydroxocomplex
ML5Xn++ OH-ML5(OH)n+ X-
Anation(Replacement of coordinated water): Reverse of acid hydrolysisML5(OH2)n++ X-ML5Xn-1+ H2O
Water Exchange reactions
Solovolysis
Types of octahedral substitution reactions
canbeputintofourclasses.Ɇe.g. alkalaimetals and larger alkaline earths
-Ǣ γ ͳ-5to 108sec-1
Ɇe.g. dipositive transition metals and tri-positive lanthanides 2- Ǣ γ ͳ - ͳ-4sec-1 Ɇe.g. most of the tri-positive transition metals, Be+2and Al+33 ȋ-... "-ȌǢ γ ͳ--1to 10-9sec-1
e.g. Cr+3(d3); Co+3(LS d6); Pt+2(LS d8); Rh+2; Ru+2 causecrystalfieldtobecomelessstable.Kinetics of Octahedral Substitution
Rate measurements
ForInertSystems:
Direct chemical analysis : argentomentry
Spectrophotometric methods
quotesdbs_dbs17.pdfusesText_23[PDF] meckwell bridge convention
[PDF] med cet 2020
[PDF] med pathway mcat
[PDF] media base charts
[PDF] mediabase activator chart
[PDF] mediabase smooth jazz charts
[PDF] mediabase vs bds
[PDF] medical abbreviation
[PDF] medical abbreviation for without
[PDF] medical abbreviations and symbols
[PDF] medical abbreviations with multiple meanings
[PDF] medical coding for dummies pdf
[PDF] medical device regulation singapore
[PDF] medical evacuation protocol