 Questions and Answers – Mesomeric Effect 1. In mesomeric effect
Questions and Answers – Mesomeric Effect 1. In mesomeric effect
Answer: d. Explanation: In the mesomeric effect the electrons are transferred from multiple bonds to an atom
 INDUCTIVE AND MESOMERIC EFFECTS IN SUBSTITUTED
INDUCTIVE AND MESOMERIC EFFECTS IN SUBSTITUTED
The inductive and mesomeric effect of substituents on the electroilic charge distribution in benzene has been the subject of many theoretical treatments. In the
 [1961] Smith. 16. Xteric Repression of the Mesomeric Effect in
[1961] Smith. 16. Xteric Repression of the Mesomeric Effect in
Xteric Repression of the Mesomeric Effect in Derivatives of NN-Di- methylaniline. The Mesomeric Moments of Amino- Methylamino-
 BSc Chemistry
BSc Chemistry
There are four types of electronic effects mainly viz. inductive effect
 Mesomeric effect & its application.pdf
Mesomeric effect & its application.pdf
Mesomeric effect. and. The relay of. A electrons from m the side of the another in a molecule side to. Conjugated system is effect. It is a mesomenic ermament
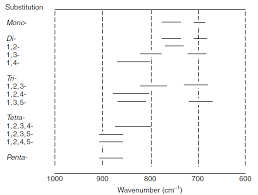 Factors affecting vibrational frequencies and IR Spectroscopy of
Factors affecting vibrational frequencies and IR Spectroscopy of
The mesomeric effect involves the electrons in the pie and nonbonding orbitals and it operates in general opposite to the inductive effect. These effects cannot
 hammetts substitution constant for the methylsulphonyl group and
hammetts substitution constant for the methylsulphonyl group and
mesomeric effect -M4) if on a conjugated place in R
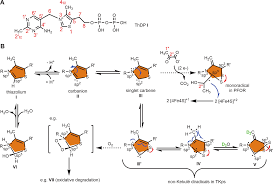 The Mesomeric Effect of Thiazolium on non‐KekulØ Diradicals in
The Mesomeric Effect of Thiazolium on non‐KekulØ Diradicals in
Abstract: It is theoretically plausible that thiazolium meso- merizes to congeners other than carbene in a low effective dielectric binding site; especially
 mesomeric effect - IUPAC Gold Book
mesomeric effect - IUPAC Gold Book
mesomeric effect. Also contains definition of: resonance effect. The effect (on reaction rates ionization equilibria
 Mesomeric effect
Mesomeric effect
11-Mar-2021 The mesomeric effect in chemistry is a property of substituents or functional groups in a chemical compound. It is defined as the polarity ...
 Questions and Answers – Mesomeric Effect 1. In mesomeric effect
Questions and Answers – Mesomeric Effect 1. In mesomeric effect
Answer: d. Explanation: In the mesomeric effect the electrons are transferred from multiple bonds to an atom
 Mesomeric effect & its application.pdf
Mesomeric effect & its application.pdf
effect. It is a mosomeric permament offer an extends in the molecule as. The structure. ; due to this offect is called reso or arising out resonating.
 BSc Chemistry
BSc Chemistry
Mesomeric Effects. TABLE OF CONTENT. 1. Learning outcomes. 2. Introduction. 3. Types of Electronic effects. 3.1 Inductive Effect. 3.2 Mesomeric or resonance
 IR Spectroscopy (Module-1I) Lecture Notes: Dr. Diksha Katiyar
IR Spectroscopy (Module-1I) Lecture Notes: Dr. Diksha Katiyar
effect including inductive effect mesomeric effect
 Hammett's substitution constant for the methylsulphonyl group
Hammett's substitution constant for the methylsulphonyl group
negative mesomeric effect and that its sulphur atom can extend the octet of electrons to a decet structure. Recently the structure of the sulphonyl group
 NOTE ON THE INDUCTIVE AND MESOMERIC EFFECTS IN
NOTE ON THE INDUCTIVE AND MESOMERIC EFFECTS IN
determined only by the mesomeric effect the negative charges in ortho and para by both effects. The ortho-para orienting character of the substituent is
 The Mesomeric Effect of Thiazolium on nonâ•Kekulé Diradicals in
The Mesomeric Effect of Thiazolium on nonâ•Kekulé Diradicals in
The Mesomeric Effect of Thiazolium on non-KekulØ Diradicals in. Pichia stipitis Transketolase. Ning-Shian Hsu+ Yung-Lin Wang+
 Factors affecting vibrational frequencies and IR Spectroscopy of
Factors affecting vibrational frequencies and IR Spectroscopy of
To understand the effect of bond strength on absorption frequency The mesomeric effect involves the electrons in the pie and nonbonding orbitals and it ...
 The Mesomeric Effect of Thiazolium on nonâ•Kekulé Diradicals in
The Mesomeric Effect of Thiazolium on nonâ•Kekulé Diradicals in
The Mesomeric Effect of Thiazolium on non-KekulØ Diradicals in. Pichia stipitis Transketolase. Ning-Shian Hsu+ Yung-Lin Wang+
 [PDF] Mesomeric effect - LS College Muzaffarpur
[PDF] Mesomeric effect - LS College Muzaffarpur
11 mar 2021 · The effect is used in a qualitative way and describes the electron withdrawing or releasing properties of substituents based on relevant
 [PDF] Mesomeric effect & its applicationpdf
[PDF] Mesomeric effect & its applicationpdf
Mesomeric effect and The relay of A electrons from m the side of the another in a molecule side to Conjugated system is effect It is a mesomenic
 (PDF) Resonance and the Mesomeric Effect - ResearchGate
(PDF) Resonance and the Mesomeric Effect - ResearchGate
9 oct 2022 · PDF Resonance Effect: The polarity induced in a molecule by the interaction of a lone pair of electrons with a pi bond or the interaction
 [PDF] BSc Chemistry - e-PG Pathshala
[PDF] BSc Chemistry - e-PG Pathshala
There are four types of electronic effects mainly viz inductive effect mesomeric (or resonance) effect electromeric effect and hyperconjugative effect Of
 [PDF] 1 Basic Concepts - Wiley-VCH
[PDF] 1 Basic Concepts - Wiley-VCH
The first is the inductive effect and the second is the mesomeric effect For a student who knows and understands these two concepts well it will be easier
 [PDF] Questions and Answers – Mesomeric Effect - CUTM Courseware
[PDF] Questions and Answers – Mesomeric Effect - CUTM Courseware
The mesomeric effect is a permanent effect and operates in compounds containing at least one double bond 3 The phenomenon in which 2 or more structures
 Mesomeric Effect - Definition Types Difference between - Byjus
Mesomeric Effect - Definition Types Difference between - Byjus
In simple terms we can describe that the mesomeric effect occurs when ? electrons move away from or towards a substituent group in a conjugated orbital system
 [PDF] Block – 2: Organic reaction mechanism - UOU
[PDF] Block – 2: Organic reaction mechanism - UOU
There are four types of electronic effects mainly viz inductive effect mesomeric (or resonance) effect electromeric effect and hyperconjugative effect
 [PDF] CONCEPTS IN ORGANIC CHEMISTRY - KEA
[PDF] CONCEPTS IN ORGANIC CHEMISTRY - KEA
Electromeric effect is defined as the complete transfer of electrons of a multiple bond towards one of the bonded atoms at the demand of an
 [PDF] 1The Group which shows +M effect is 2) -NO 3) -OH 4 - KEA
[PDF] 1The Group which shows +M effect is 2) -NO 3) -OH 4 - KEA
-E effect involves complete transfer of pi bond present in carboxyl group by attacking reagents CN- 1)Mesomeric effect 2)Inductive effect
What is the definition of the mesomeric effect?
In chemistry, the mesomeric effect (or resonance effect) is a property of substituents or functional groups in a chemical compound. It is defined as the polarity produced in the molecule by the interaction of two pi bonds or between a pi bond and lone pair of electrons present on an adjacent atom.What are the two types of Mesomeric effects?
If a substituent with a double bond or nonbonding electrons is directly attached to a conjugated system, the electron density and, as a result, the chemical shift will change. Further, there are two types of mesomeric effects: +M effect and -M effect according to types of mesomeric effects notes.What is an example of -+ M effect?
If the ? electrons move away from the group and towards the rest of the molecule, the effect is called a +M effect . An example is the donation of electrons from an amino group into a benzene ring, putting ?? charges on the ortho and para positions.- The main difference between resonance and the mesomeric effect is that the resonance effect describes how a molecule's lone pair of electrons and bond pair of electrons determine its chemical structure, whereas the mesomeric effect describes how a molecule's chemical structure is stabilized by using a functional group.
[PDF] messari exchange
[PDF] messiaen complete organ works
[PDF] messiaen complete organ works olivier latry
[PDF] messiaen latry organ works
[PDF] messiaen nightingale
[PDF] messiaen quartet for the end of time best recording
[PDF] messiaen quartet wiki
[PDF] messiaen quatuor pour la fin
[PDF] meta 1 coin sec
[PDF] meta 1 coin trust
[PDF] meta 1 coin value
[PDF] meta coins
[PDF] meta investment bank
[PDF] meta1 exchange
