Thermal Properties of Matter
Thermal Properties of Matter
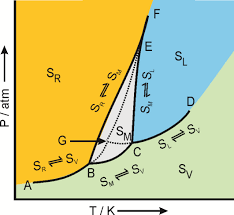
For example the triple point of water is represented by the temperature 273.16 K and pressure 6.1110–3 Pa. (a). (b). Pressure-temperature phase diagrams for (a)
 MODULE 5: DISTILLATION
MODULE 5: DISTILLATION
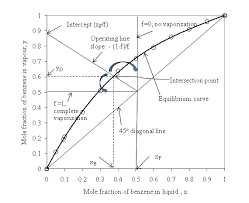
temperature phase diagram is useful in the analysis of solution behaviour. A mixture of 40 mole % isopropanol in water is to be batch-distilled at 1 atm until.
 Water Raising Monoset pump-hindi
Water Raising Monoset pump-hindi
These are horizontal centrifugal self priming mono set pumps which are driven by 3- phase 50 Hz. js[kksss fp= SCHEMATIC DIAGRAM. 7 vkj
 Heat treatment and properties of iron and steel
Heat treatment and properties of iron and steel
Phase diagram for carbon steels. ferrite and pearlite (fig. 5 B and C). The DISTANCE'FROM WATER QUENCHED END. 1/16 IN. Figure 23. Relation between ...
 Chiller System Design and Control / Applications Engineering Manual
Chiller System Design and Control / Applications Engineering Manual
٠١/١١/٢٠١١ reductions during the “value engineering” phase of a project. ... Also known as leaving-chilled-water or leaving-evaporator- water; chilled water ...
 Operational Guidelines for the Implementation of Jal Jeevan Mission
Operational Guidelines for the Implementation of Jal Jeevan Mission
٢١/١٢/٢٠١٩ water quality water-borne disease
 Phase Diagrams for Three Component Mixtures in Pharmaceuticals
Phase Diagrams for Three Component Mixtures in Pharmaceuticals
Water titra- tion method was used for the phase diagram construction and visual inspection was performed. Observed phases after each addition were tabulated.
 Guidelines on Water Purification By Reverse Osmosis(RO)
Guidelines on Water Purification By Reverse Osmosis(RO)
The feed water or permeate flows through the system more rapidly than during the production phase. 12.0 SCHEMATIC DIAGRAM OF RO PLANT : Feed Pump. Anti ...
 Probing the Heterogeneity of Ionic Liquids in Solution through
Probing the Heterogeneity of Ionic Liquids in Solution through
٠٦/١٠/٢٠١٨ When IL is added at various concen- trations in the phenol-water system the concentration-depend- ent effect on the phase diagram is observed.
 Properties of Pure Substances Pure Substance Phases of a Pure
Properties of Pure Substances Pure Substance Phases of a Pure
Water expands upon freezing. Fig. 3: phase diagram of pure substances. There are two ways that a substance can pass from solid phase to vapor phase i) it
 FORMATION OF COMPOUNDS WITH CONGRUENT MELTING
FORMATION OF COMPOUNDS WITH CONGRUENT MELTING
where double formula are used for ferric chloride in order to avoid a fractional number of molecules of water of crystallization. The phase diagram of this.
 Water Raising Monoset pump-hindi
Water Raising Monoset pump-hindi
ceiling of the coaches to supply water in toilets & wash basins. driven by 3- phase 50 Hz. 415 V ac supply. ... js[kksss fp= SCHEMATIC DIAGRAM.
 Lead-silver system and its explanation: Phase Diagram
Lead-silver system and its explanation: Phase Diagram
solid and liquid phase of lead are D in equilibrium at this point. Now if silver is added in it
 Time Temperature Transformation (TTT) Diagrams
Time Temperature Transformation (TTT) Diagrams
water. The microstructure of each sample is studied using metallographic techniques. The type as well as quantity of phases
 Chapter 6 Phase transitions
Chapter 6 Phase transitions
phase transition. The appropriate variables for phase diagram of water are the pressure P and the temper- ature T. critical point : The first-order phase
 Teach Yourself Phase Diagrams and Phase Transformations
Teach Yourself Phase Diagrams and Phase Transformations
11 mars 2009 What happens at a given temperature and composition is determined by the thermodynamics of mixing sugar and water. Minimising free energy ...
 Chapter 9: Phase Diagrams
Chapter 9: Phase Diagrams
solubility limit at 20°C? Answer: 65 wt% sugar. If Co < 65 wt% sugar: syrup. If Co > 65 wt% sugar: syrup + sugar. 65. Sucrose/Water Phase Diagram.
 19 The Phase Rule
19 The Phase Rule
DERIVATION OF THE PHASE. RULE. ONE–COMPONENT SYSTEM. PHASE DIAGRAMS. POLYMORPHISM. EXPERIMENTAL DETERMINATION. OF TRANSITION POINT. THE WATER SYSTEM.
 The Phase Diagram of a DeepPotential WaterModel - arXivorg
The Phase Diagram of a DeepPotential WaterModel - arXivorg
FIG 1: Phase diagram of water (a1) DP model (red solid lines) and experiment (gray solid lines) for T
How to draw a phase diagram?
- Draw a vertical line on the phase diagram at the appropriate composition and determine the point at which it intersects the liquid composition line, which is marked in red. (b) Draw a horizontal tie line and deduce the compositon of the vapour from the blue line.
What is the liquid phase?
- The liquid phase exists at high pressure and high temperature, and is therefore represented by area B. Standard ambient temperature and pressure (SATP) corresponds to 1 atm = 1.0325 bar and 25 ?C = 298 K. This point falls within area C, which corresponds to the gas or vapour phase. The phases that occur depend upon the pressure of the sample.
What are the phases of a substance?
- The phases that occur depend upon the pressure of the sample. At 35 bar, the substance exists as a liquid (area B) at 250 K, but becomes a solid (area A) at 150 K. However, at 5 bar, the substance exists as a vapour (area C) at 250 K, and sublimes to become a solid (area A) at 100 K.
How do you find the equilibrium between two solid phases?
- The equilibrium between two solid phases is described by the Clapeyon equation. Since you have the two densities then the volume change for the phase Use Equation 17.11, which shows how the electrostatic potential energy between two charges varies with separation. 2 PE=40 0. Mg2+ ions 16.
[PDF] phase diagram of water triple point
[PDF] phase diagram of water vs co2
[PDF] phase diagram of water vs other substances
[PDF] phase diagram of water worksheet
[PDF] phase of a discrete signal
[PDF] phases of water
[PDF] phatic function of language examples
[PDF] phd economics
[PDF] phd economics europe
[PDF] phd in disaster management in ignou
[PDF] phe budget 2020
[PDF] phelan
[PDF] phemius pronunciation
[PDF] phenol acetic anhydride reaction mechanism