 Properties of Pure Substances Pure Substance Phases of a Pure
Properties of Pure Substances Pure Substance Phases of a Pure
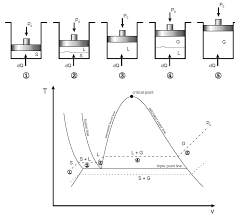
diagram for a pure substance is very similar to that of a T-v ... This is called phase diagram since all three phases are separated from each other by three.
 1) A phase diagram is a graph of pressure vs. temperature that
1) A phase diagram is a graph of pressure vs. temperature that
other substances like a liquid). 7. Page 2. Pressure (kPa). Phase Diagram of ... Use the phase diagram for water below to answer the following questions. 10000.
 Solutions Manual for Thermodynamics and Chemistry
Solutions Manual for Thermodynamics and Chemistry
Jun 9 2020 Figure 27 Temperature–composition phase diagram for the binary system of water. (A) and phenol (B) at 1 bar.a Only liquid phases are present.
 Chocolate Experiment
Chocolate Experiment
Although chocolate also undergo the phase changes as temperature changes similarly to water or any other substance
 Skill Practice 4 - Phase Diagrams
Skill Practice 4 - Phase Diagrams
Phase diagram for substance A: Liquid. Pressure (kPa). Solid. Name: Date: Hour: 101.325 One of the substances behaves more like most other substances. Which ...
 Calculation of a temperature-volume phase diagram of water to
Calculation of a temperature-volume phase diagram of water to
During isochoric freezing an aqueous system is confined within a rigid
 ChemQuest 9 Information: Phase diagrams Critical Thinking Questions
ChemQuest 9 Information: Phase diagrams Critical Thinking Questions
The triple point of a substance is the conditions under which a solid liquid and gas all exist in equilibrium. On the phase diagram of water
 Properties of Pure Substances Pure Substance Phases of a Pure
Properties of Pure Substances Pure Substance Phases of a Pure
Water expands upon freezing. Fig. 3: phase diagram of pure substances. There are two ways that a substance can pass from solid phase to vapor phase i
 Calculation of a temperature–volume phase diagram of water to
Calculation of a temperature–volume phase diagram of water to
17 Jul 2022 Phase diagrams are integral to the application and interpretation of materials thermodynamics and none is more ubiquitous than the common ...
 Ch. 11: Liquids and Intermolecular Forces
Ch. 11: Liquids and Intermolecular Forces
Explain how water's phase diagram differs from most other substances and why. ? Describe how the molecular arrangements characteristic of nematic smectic
 Solutions Manual for Thermodynamics and Chemistry
Solutions Manual for Thermodynamics and Chemistry
9 Jun 2020 11 Reactions and Other Chemical Processes. 58. 12 Equilibrium Conditions in Multicomponent Systems. 77. 13 The Phase Rule and Phase Diagrams.
 Lecture 12
Lecture 12
1 Oct 2018 Example: water vs. temperature. ... O vs. other most other substances – different slope of liquid/solid. ... Phase diagram Helium.
 THERMODYNAMICS PROPERTIES OF PURE SUBSTANCES Pure
THERMODYNAMICS PROPERTIES OF PURE SUBSTANCES Pure
Figure 3: T-v diagram representing phase change for water at constant pressure. This concept can be applied to pure substance other than water. Vapor. Saturated.
 Chapter 1 INTRODUCTION AND BASIC CONCEPTS
Chapter 1 INTRODUCTION AND BASIC CONCEPTS
P-v diagram of a substance that expands on freezing (such as water). At triple-point pressure and temperature a substance exists in three phases in
 Phase Diagrams of Ordinary Water Substance
Phase Diagrams of Ordinary Water Substance
grams is that the ranges in values of the p and V variables are phase diagram of the principal solid phases of water with broken.
 4A Phase diagrams of pure substances
4A Phase diagrams of pure substances
20 Apr 2019 9 shows that water has one liquid phase but many different solid phases other than ordinary ice ('ice I'). Some of these phases melt at high ...
 Lecture 14 Equilibrium between phases (Ch5)
Lecture 14 Equilibrium between phases (Ch5)
The generic phase diagram of a substance in the P-Tcoordinates is shown above Every point of this diagram is an equilibrium state; different states of the system in equilibrium are called phases The lines dividing different phases are called the coexistence curves
 Chapter 9: Phase Diagrams - University of Washington
Chapter 9: Phase Diagrams - University of Washington
Phase Diagrams: composition of phases Rule 2: If we know T and Co then we know: --the composition of each phase T(°C)• Examples: o= 35 wt NiTAAt TA= 1320°C: 1300L (liquid) Only Liquid (L)CL= Co( = 35 wt Ni)TBAt TD= 1190°C: 1200 Only Solid (Cu-Ni system tie line ? (solid) D ?= Co( = 35 wt Ni) 20303235 = 1250°C: CLCo T ) ? D
 Water Phase Diagram Density of Water in its Three Phases
Water Phase Diagram Density of Water in its Three Phases
The P?T or Phase Change Diagram This is called phase diagram since all three phases are separated from each other by three lines Most pure substances exhibit the same behavior One exception is water Water expands upon freezing Fig 3: phase diagram of pure substances
 Interpreting Phase Diagrams - University of Houston
Interpreting Phase Diagrams - University of Houston
The diagram tells us that a finite drop in temperature requires the solid solution phase to change composition in order to be in equilibrium with the liquid This means that the solid solution phase must be homogeneous - the same chemical composition throughout the solid solution phase
 Phase diagram of water - Columbia University
Phase diagram of water - Columbia University
water molecules are cohesive - 'stick' to one another and to other polar molecules Name surface tension (dynes/cm at 20oC) Water 73 Methanol 22 Ethanol 22 Ether 17 insect on water The surface tension makes air-water boundaries distinctive microhabitats
 Searches related to phase diagram of water vs other substances filetype:pdf
Searches related to phase diagram of water vs other substances filetype:pdf
The phase diagram of water Density change Triple points The ice phases Phase diagrams Phase diagrams show the preferred physical states of matter at different temperatures and pressure Within each phase the material is uniform with respect to its chemical composition and physical state
What is unusual about the phase diagram for water?
- Well, it's one way the water phase diagram is different. Water has a unique structure that makes the liquid denser than the solid. Density is how much mass is in a certain volume.
What is triple point phase diagram of water?
- Triple point is the intersection on a phase diagram where three phases coexist in equilibrium. The most important application of triple point is water, where the three-phase equilibrium point consists of ice, liquid, and vapor.
What is water in its normal phase?
- Water occurs as a liquid on the surface of Earth under normal conditions, which makes it invaluable for transportation, for recreation, and as a habitat for a myriad of plants and animals. The fact that water is readily changed to a vapour (gas) allows it to be transported through the atmosphere from the oceans to inland areas where it condenses and, as rain , nourishes plant and animal life.
[PDF] phase of a discrete signal
[PDF] phases of water
[PDF] phatic function of language examples
[PDF] phd economics
[PDF] phd economics europe
[PDF] phd in disaster management in ignou
[PDF] phe budget 2020
[PDF] phelan
[PDF] phemius pronunciation
[PDF] phenol acetic anhydride reaction mechanism
[PDF] phenol acidity
[PDF] phenol pka
[PDF] phenols reactions ppt
[PDF] pheromonal communication in ants
