 Standard Reduction Potentials of Half-Cells - F2(g) + 2e- 2F-(aq) +
Standard Reduction Potentials of Half-Cells - F2(g) + 2e- 2F-(aq) +
Standard Reduction Potentials of Half-Cells. (Ionic concentrations are at 1M in water @ 250 C). Oxidizing Agents. Reducing Agents. E0 (Volts). F2(g) + 2e-. 2F-(
 Exercise 18.2 - Standard Cell Potentials - Answers
Exercise 18.2 - Standard Cell Potentials - Answers
DIRECTIONS: Write the oxidation and reduction half reactions. Calculate the standard cell potential for the following electrochemical cells. 3. Ag+(aq) + Fe(s)
 A student is given a standard galvanic cell represented above
A student is given a standard galvanic cell represented above
https://secure-media.collegeboard.org/digitalServices/pdf/ap/apcentral/ap14_chemistry_q3.pdf
 Chapter 18: Electrochemistry
Chapter 18: Electrochemistry
Standard cell potential : The cell potential under standard state conditions [ions] = 1 M
 APPENDIX H Standard Reduction Potentials*
APPENDIX H Standard Reduction Potentials*
Caroli Tables of Standard Electrode Potentials (New York: Wiley
 Standard Electrode Potentials and Temperature Coefficients in
Standard Electrode Potentials and Temperature Coefficients in
Key words: electrochemical cell reaction; equilibrium constant; half-reaction; standard electrode A standard electrode potential E" is defined as the po-.
 Solutions for Cell Potentials Extra Exercises
Solutions for Cell Potentials Extra Exercises
For each of the following standard cells write the cell notation
 Cell Potential
Cell Potential
18-mar 2020 By convention
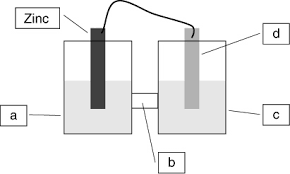 Test4 ch19 Electrochemistry Practice-answers-Marked
Test4 ch19 Electrochemistry Practice-answers-Marked
Using the Table of Standard Reduction Potentials table shown above what is the standard cell potential for an electrochemical cell that has iron (Fe) and
 Section 10.2: Standard Reduction Potentials
Section 10.2: Standard Reduction Potentials
Analysis: Use the equation !E°r (cell) = E°r (cathode) " E°r (anode) to calculate the standard cell potential. Solution: The half-cell reaction with more
 Standard Reduction Potentials of Half-Cells - F2(g) + 2e- 2F-(aq) +
Standard Reduction Potentials of Half-Cells - F2(g) + 2e- 2F-(aq) +
Standard Reduction Potentials of Half-Cells. (Ionic concentrations are at 1M in water @ 250 C). Oxidizing Agents. Reducing Agents. E0 (Volts). F2(g) + 2e-.
 Test4 ch19 Electrochemistry Practice-answers-Marked
Test4 ch19 Electrochemistry Practice-answers-Marked
For the cell shown the standard reduction potentials are +0.80 V for Ag+ and standard reduction potential of Cu2+ ? Cu is +0.34V
 Effect of electrode electronegativity on standard cell potential
Effect of electrode electronegativity on standard cell potential
The voltage produced by a galvanic cell is calculated by adding the standard cell potentials. (standard refers to the concentration of electrolytic solution at
 Untitled
Untitled
6 nov. 2017 Given that the standard reduction potential of Zn²+ to Zn(s) is ... 2) The standard cell potential is 1.46 V for a voltaic cell based on the ...
 A student is given a standard galvanic cell represented above
A student is given a standard galvanic cell represented above
https://secure-media.collegeboard.org/digitalServices/pdf/ap/apcentral/ap14_chemistry_q3.pdf
 Electrochemical Cells Worksheet
Electrochemical Cells Worksheet
Calculate the standard cell potential produced by a galvanic cell consisting of a nickel electrode in contact with a solution of Ni2+ ions and a silver
 Untitled
Untitled
DIRECTIONS: Write the oxidation and reduction half reactions. Calculate the standard cell potential for the following electrochemical cells.
 Untitled
Untitled
Given the standard reduction potentials. E = 0.34 V. E' -0.80 V. A). B). Cu²+ + 2e ? Cu. Ag¹. +e - Ag calculate the cell potential
 Untitled
Untitled
Calculate the standard cell potential produced by a voltaic cell consisting of a nickel electrode in contact with a solution of Ni2+ ions and a silver
 CHEM1612 2014-N-12 November 2014 • In the electrolytic
CHEM1612 2014-N-12 November 2014 • In the electrolytic
12 nov. 2014 Calculate the electromotive force of the cell at 25 °C. Marks. 5. The standard reduction reactions and potentials for the two half cells are:.
 [PDF] Chapter 18
[PDF] Chapter 18
The important characteristics of the standard electrode potential is: 1 It is a relative quantity---the potential of an electrochemical cell in which the
 [PDF] Cell Potential
[PDF] Cell Potential
18 mar 2020 · The standard cell potential equals to the difference between the abilities of the two electrodes to act as reducing agents E° cell = E°
 [PDF] APPENDIX H Standard Reduction Potentials* - CSUN
[PDF] APPENDIX H Standard Reduction Potentials* - CSUN
1964 and 1971); G Milazzo and S Caroli Tables of Standard Electrode Reduction potentials for 1 200 free radical reactions are given by P Wardman
 [PDF] Electrode Potentials
[PDF] Electrode Potentials
The standard electrode potential Eo of a half-reaction is defined as its electrode potential when the activities of the reactants and products are all unity
 [PDF] Standard Reduction Potentials at 25°C - Half-Reaction E° (V)
[PDF] Standard Reduction Potentials at 25°C - Half-Reaction E° (V)
Standard Reduction Potentials at 25°C Half-Reaction E° (V) Ag + (aq) + e - ? Ag (s) +0 799 AgBr (s) + e - ? Ag (s) + Br
 [PDF] CRC Handbook of Chemistry and Physics
[PDF] CRC Handbook of Chemistry and Physics
Table 3 lists only those reduction potentials which have E° negative with respect to the standard hydrogen electrode In Table 3 the reactions are listed in
 [PDF] Chapter 18: Electrochemistry
[PDF] Chapter 18: Electrochemistry
Cell Notation (shorthand for describing a galvanic cell): Standard Reduction Potentials Electrical current flows from the anode to the cathode due to a
 [PDF] Electrode Potential/Voltage
[PDF] Electrode Potential/Voltage
the anode of an electrochemical cell: E°cell = E°anode – E°cathode 2 How to calculate the Standard Cell Potential for a redox reaction:
 [PDF] Topic 9 Electrochemistry
[PDF] Topic 9 Electrochemistry
23 jan 2018 · 17 2 Standard Reduction Potentials ? Reaction in galvanic cell is oxidation-reduction split into two half-reactions
What is the standard cell potential?
A cell's standard state potential is the potential of the cell under standard state conditions, which is approximated with concentrations of 1 mole per liter (1 M) and pressures of 1 atmosphere at 25oC.What is standard cell potential summary?
The standard cell potential is the potential difference between the cathode and anode. For more information view Cell Potentials. The standard potentials are all measured at 298 K, 1 atm, and with 1 M solutions.What is the formula for the standard cell potential?
The overall cell potential can be calculated by using the equation E0cell=E0red?E0oxid.- Electromotive Force (EMF) has been measured to be 1.100 V. A concentration of 1 M in an ideal solution is defined as the standard condition, and 1.100 V is thus the standard electromotive force, DEo, or standard cell potential for the Zn?Cu galvanic cell.