 Reaction rate and rate constant of the hydrolysis of ethyl acetate with
Reaction rate and rate constant of the hydrolysis of ethyl acetate with
Hydrolysis of an ester such as acetate in presence of a mineral acid: Hydrolysis is a chemical decomposition involving breaking of a bond and the addition
 Determination of the Expression Rate of Ethyl Acetate Hydrolysis
Determination of the Expression Rate of Ethyl Acetate Hydrolysis
So the hydrolysis of ethyl acetate to produce the sodium salt of acetic acid (NaOAc)
 Concept laboratory preparation
Concept laboratory preparation
https://fctemis.org/notes/487_Concept
 Researching Chemistry (SCQF level 7) Unit code: FE4J 13 Course
Researching Chemistry (SCQF level 7) Unit code: FE4J 13 Course
♢ Preparation of benzoic acid by hydrolysis of ethyl benzoate. ♢ Preparation of ethyl ethanoate. ♢ Preparation of cyclohexene from cyclohexanol. A video
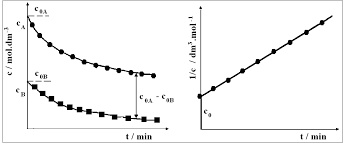 Exercise 6 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE
Exercise 6 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE
Determine the rate constant and the activation energy of the alkaline hydrolysis of ethyl 3 Pipette VHCl= 5 ml solution of hydrochloric acid (c = 0.04 mol dm- ...
 4.5 Carboxylic acids and their derivatives
4.5 Carboxylic acids and their derivatives
ethanoic acid ethanol ethyl ethanoate. Acid chlorides. Prepared by the reaction between a carboxylic acid and phosphorus pentachloride PCl5. Dry conditions
 FACTFILE: GCE CHEMISTRY
FACTFILE: GCE CHEMISTRY
For example ethanenitrile would produce ammonium ethanoate via ethanamide. Acid hydrolysis of nitrile ethyl ethanoate sodium ethanoate ethanol. H2O. CH3CN.
 1 Ethanoic acid and ethanol react together to form the ester ethyl
1 Ethanoic acid and ethanol react together to form the ester ethyl
(f) (i) Complete the equation below for the alkaline hydrolysis of ethyl ethanoate using sodium hydroxide. State symbols are not required. (1). 3. CH COOCH. 2.
 Topic 3 – Chemical Structure and Bonding
Topic 3 – Chemical Structure and Bonding
The hydrolysis of ethyl ethanoate is a reversible reaction. The equation for He found that 2.0 mol of ethanoic acid had formed. The information in the ...
 Chapter 5 Carboxylic Acids and Esters
Chapter 5 Carboxylic Acids and Esters
– Ethanoic acid/acetic acid is the main ingredient in Esters may be broken apart under acidic conditions by water (a hydrolysis reaction) to form a carboxylic ...
 [PDF] Exercise 6 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE
[PDF] Exercise 6 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE
Exercise 6 KINETICS OF THE HYDROLYSIS OF ETHYL ACETATE 3 Pipette VHCl= 5 ml solution of hydrochloric acid (c = 0 04 mol dm-3) into a clean and
 [PDF] IV SEMMESTER
[PDF] IV SEMMESTER
KINETICS OF ACID HYDROLYSIS OF AN ESTER AIM: To determine the rate constant of the hydrolysis of Ethyl acetate using an acid as a catalyst PRINCIPLE:
 [PDF] Temperature Dependence of Ester Hydrolysis in Water
[PDF] Temperature Dependence of Ester Hydrolysis in Water
The rate of the hydroxyl-promoted hydrolysis of ethyl acetate has been Arrhenius activation energy of the alkaline and acid hydrolysis shows an
 [PDF] Effect of Ion Exchange Resin Catalyst on Hydrolysis of Ethyl Acetate
[PDF] Effect of Ion Exchange Resin Catalyst on Hydrolysis of Ethyl Acetate
alternative heterogeneous catalyst for hydrolysis reaction of ethyl acetate The resin had to be washed using sulphuric acid to give an H+ charge on the