 J. J. Thomson and The Electron: 1897–1899 An Introduction
J. J. Thomson and The Electron: 1897–1899 An Introduction
Jul 19 2023 Some difficulties in executing the experiments complicated matters still further. Because the cathode rays ionized the residual gas in the tube
 Untitled
Untitled
Discovery of the Electron: J. J. Thomson's Experiment. Many studies of The cathode-ray tube used by J. J. Thomson (the apparatus is shown in. Figure ...
 Untitled
Untitled
Y. Page 2. 294. Prof. J. J. Thomson on Cathode Rays. unobserved phenomena in the æther of whose laws we are ignorant. The following experiments were made to
 Atomic Theory Timeline
Atomic Theory Timeline
J. J. Thomson. 1896. Robert Millikan. 1909. Ernest Rutherford. 1909. Greek 95). Discovered atoms have negative particles. (electrons) using a cathode ray tube ...
 ATOMIC STRUCTURE
ATOMIC STRUCTURE
Cathode-ray Tubes. Page 11. JJ Thomson – His Experiment 1897. ○ watch JJ Thomson's cathode ray tube · experiment. Quick Facts: Born: December 181856 Cheetham
 J J Thomson and the discovery of the electron
J J Thomson and the discovery of the electron
The experiment relied on electrostatic deflection of cathode rays. Hertz's Thomson appears to have designed his cathode ray tube of. 1897 with this in ...
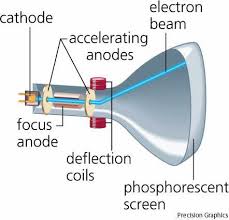
 Measurement of Charge-to-Mass (e/m) Ratio for the Electron
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
J.J. Thomson first measured the charge-to-mass ratio of the fundamental particle of charge in a cathode ray tube in 1897. A cathode ray tube basically
 Untitled
Untitled
How did J.J. Thomson know that electrons had a negative charge? Answer: His experiments with the cathode ray tube.
 Philosophical Magazine Series 5 XL. Cathode Rays
Philosophical Magazine Series 5 XL. Cathode Rays
J. J. Thomson on Cathode Ray~. 299" one minute is the quantity required to Some experiments were made with a tube similar to that shown in fig. 2 with ...
 Atoms: Atom is the smallest indivisible particle of the matter. Atom is
Atoms: Atom is the smallest indivisible particle of the matter. Atom is
J. J. Thomson. (1869). Goldstein (1886). Chadwick (1932). Nature of charge Cathode ray discharge tube experiment: A cathode ray discharge tube made of glass ...
 Electrons as Particles Thomsons Cathode Ray Tube Experiment
Electrons as Particles Thomsons Cathode Ray Tube Experiment
Thomson's Cathode Ray Tube Experiment. Worksheet Key. Background. Joseph John Thomson was an Englishman. While studying mathematics at Trinity.
 ATOMIC STRUCTURE
ATOMIC STRUCTURE
JJ Thomson – His Experiment 1897. ? watch JJ Thomson's cathode ray tube · experiment. Quick Facts: Born: December 181856 Cheetham Hill Manchester
 J. J. Thomson and The Electron: 1897–1899 An Introduction
J. J. Thomson and The Electron: 1897–1899 An Introduction
Jul 19 2022 Some difficulties in executing the experiments complicated matters still further. Because the cathode rays ionized the residual gas in the tube
 The History of the Atom
The History of the Atom
Scientist: J.J Thomson. J.J Thomson was a physicist who is credited for discovering the electron. He used his research on cathode ray tube technology in
 Measurement of Charge-to-Mass (e/m) Ratio for the Electron
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
J.J. Thomson first measured the charge-to-mass ratio of the fundamental particle of charge in a cathode ray tube in 1897. A cathode ray tube basically
 Thomsons Cathode Ray Tube Experiment
Thomsons Cathode Ray Tube Experiment
1. The simulation will open with a model of a cathode ray tube used by J.J.. Thomson in his experiment. 2. Sketch a picture of the cathode ray tube.
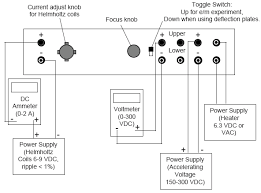
 Charge to Mass of Electron
Charge to Mass of Electron
THE J. J. THOMSON EXPERIMENT. TABLE OF CONTENTS Thomson subjected the cathode rays in his tube to electric and magnetic fields at the same.
 J J Thomson and the discovery of the electron
J J Thomson and the discovery of the electron
this experiment was performed two months after Thomson first announced that cathode rays were very small negatively charged particles. So why was it.
 Untitled
Untitled
Discovery of the Electron: J. J. Thomson's Experiment The cathode-ray tube used by J. J. Thomson (the apparatus is shown in. Figure 3-1) is typical of ...
 2.4 Early Experiments to Characterize the Atom
2.4 Early Experiments to Characterize the Atom
J. J. Thomson (1856-1940) - postulated the existence of negatively charged particles called electrons using cathode ray tubes. •