 TOLLENS REAGENT MSDS CAS No
TOLLENS REAGENT MSDS CAS No
14-Mar-2018 If you feel unwell seek medical advice. Page 3. TOLLEN'S REAGENT. Safety Data Sheet www.lobachemie.
 Aldehydes Aldehydes Ketones and Carboxylic Acids Aldehydes
Aldehydes Aldehydes Ketones and Carboxylic Acids Aldehydes
Aldehydes are easily oxidised to carboxylic acids by mild oxidising reagents such as Tollens' reagent and. Fehling's reagent. These oxidation reactions are
 The mechanism of the reaction of the Tollens reagent
The mechanism of the reaction of the Tollens reagent
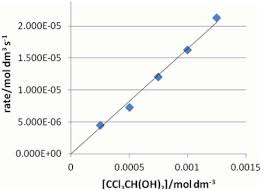
27-Dec-2011 The Tollens test for aldehydes has been used for over 100 years but no reason has been given for adding sodium hydroxide to the silver nitrate ...
 University of Utah Chemistry Demonstration: Silver Mirror (Tollens
University of Utah Chemistry Demonstration: Silver Mirror (Tollens
The Tollens reagent is used to test for the presence of aldehydes. Aldehydes are oxidized to the carboxylic acid and the [Ag(NH3)2]+ ion is reduced to
 Tollens Test
Tollens Test
11-Aug-2016 Tollens' reagent is an alkaline solution containing a silver–ammonia complex used in organic chemistry to test for the presence of aldehydes ...
 Detection of aldehydes by gold nanoparticle colorimetric array
Detection of aldehydes by gold nanoparticle colorimetric array
06-Oct-2021 Our colorimetric sensor array based on Tollens' reagent allows discrimination of ten kinds of aldehydes showing a distinct color change from ...
 THE STRUCTURAL RELATIONSHIP OF THE CARDIAC POISONS
THE STRUCTURAL RELATIONSHIP OF THE CARDIAC POISONS
We have prepared gitoxin from digitalis leaves and have studied its behavior towards both Tollens' reagent and sodium nitroprusside. In both cases definitely
 Tollens Reaction‐Based Integration of Thin Film Wall Electrodes into
Tollens Reaction‐Based Integration of Thin Film Wall Electrodes into
Tollens Reagent: The alkaline silver nitrate solution stabilized by an ammonia complex is also named Tollens reagent. In the first step the silver ions
 GLYCOSIDES & REDUCING SUGARS
GLYCOSIDES & REDUCING SUGARS
a carboxylic acid group (deprotonated) and the Ag+ reduced to Ag (s). Tollens' reagent: Test for reducing sugars. Recall that in acidic (H+) medium sugars
 Silver Mirrors
Silver Mirrors
The Tollens' reagent is generated in situ by mixing ammonium nitrate silver nitrate
 SAFETY DATA SHEET
SAFETY DATA SHEET
13 May 2019 Tollens Reagent. Product code. 204-06361. CAS RN. N/A. Manufacturer. FUJIFILM Wako Pure Chemical Corporation. 1-2 Doshomachi 3-Chome.
 The mechanism of the reaction of the Tollens reagent
The mechanism of the reaction of the Tollens reagent
27 Dec 2011 The Tollens test for aldehydes has been used for over 100 years but no ... Keywords: Tollens reagent aldehyde
 TOLLENS REAGENT MSDS CAS No
TOLLENS REAGENT MSDS CAS No
14 Mar 2018 If you feel unwell seek medical advice. Page 3. TOLLEN'S REAGENT. Safety Data Sheet www.lobachemie.
 GLYCOSIDES & REDUCING SUGARS
GLYCOSIDES & REDUCING SUGARS
Tollens' reagent: Test for reducing sugars. Recall that in acidic (H+) medium sugars exist predominantly in their cyclized form: furanoses and pyranoses.
 Aldehydes Aldehydes Ketones and Carboxylic Acids Aldehydes
Aldehydes Aldehydes Ketones and Carboxylic Acids Aldehydes
aldehydes from ketones: (i) Tollens' test: On warming an aldehyde with freshly prepared ammoniacal silver nitrate solution (Tollens' reagent) a bright.
 Quantitative paper radiochromatography using tollens reagent—I
Quantitative paper radiochromatography using tollens reagent—I
Talanta 1961. Vol. 8. pp. 809 to 816. Pergamon Press Ltd. Printed in Northern Ireknd. QUANTITATIVE PAPER RADIOCHROMATOGRAPHY. USING TOLLENS REAGENT-I.
 Tollens Test
Tollens Test
11 Aug 2016 Tollen's reagent is an alkaline solution containing a silver–ammonia complex which is used in organic chemistry to test for the presence of ...
 Student safety sheets 46 Silver and its compounds
Student safety sheets 46 Silver and its compounds
(Tollen's Reagent). EXPLOSIVE. IRRITANT. It is used for aldehyde tests and should be prepared only on a test-tube scale when needed.
 JOURNALOFCHROMATOGRAPHY 247 QUANTITATIVE PAPER
JOURNALOFCHROMATOGRAPHY 247 QUANTITATIVE PAPER
The application of Tollens reagent to radiometric determination of employing the technique of adding silver reagents to the chromatograms by dipping.
 Silver Mirrors
Silver Mirrors
The Tollens' reagent is generated in situ by mixing ammonium nitrate silver nitrate
 Department of Chemistry - The University of Utah
Department of Chemistry - The University of Utah
Department of Chemistry - The University of Utah
 BIOCHEMISTRY - MTH
BIOCHEMISTRY - MTH
If we add the Tollens’ reagent[Ag(NH 3) 2 +][OH–] to the equilibrium solution the sugar’s aldehyde group is oxidized to a carboxylic acid group (deprotonated) and the Ag+reduced to Ag (s) Tollens’ reagent: Test for reducing sugars Recallthatinacidic(H+)mediumsugarsexistpredominantlyintheircyclizedform:furanosesandpyranoses but
What Is Tollens Test?
Tollens test is generally given by compounds having aldehydic group (aldehydes,alpha-hydroxy ketones and formic acid-its -COOH behaves like a aldehydic group). It gives a white ppt of Silver (where the silver salt is reduced to silver metal and the aldehyde is oxidised to silver salt of carboxylic acid.
Table of Contents
Tollens Reagent
Tollens Reagent
Tollens Reagent refers to the chemical reagent which is used in the detection of an aldehyde functional group, an aromatic aldehyde functional group, or an alpha hydroxy ketone functional group in a given test substance. The Tollens Reagent is named after Bernhard Tollens, A German chemist who discovered this reagent and its uses. Tollens reagent i...
Alpha Hydroxy Ketone Tollens Test
Alpha hydroxy ketones are able to give a positive Tollens’ test since ?-hydroxy ketones have the ability to tautomerize to aldehydes, and the aldehyde gives the Tollens’ test. An ?-hydroxy ketone that cannot tautomerize to a aldehyde won’t give a positive Tollens’ test, like benzoin.
Tollens Reagent Preparation
Since Tollens Reagent has a relatively short shelf life, the reagent is not commercially sold. Therefore, this reagent is often prepared directly in the laboratory. One such method for the preparation of Tollens Reagent is described below:
What reagent is used in Tollens test?
Tollens’ test uses a reagent known as Tollens’ reagent, which is a colorless, basic, aqueous solution containing silver ions coordinated to ammonia [ A g ( N H 3) 2 +]. It is prepared using a two-step procedure. Step 1: Aqueous silver nitrate is mixed with aqueous sodium hydroxide.
What happens when an aldehyde is introduced to the Tollens reagent?
When an aldehyde is introduced to the Tollens reagent, two things occur: The aldehyde is oxidized by the Tollens reagent and forms a carboxylic acid. This reaction can be written as follows: The silver ions present in the Tollens reagent are reduced into metallic silver. Generally, the Tollens Test is carried out in clean test tubes made of glass.
Why is there excess ammonia in Tollens reagent?
When making Tollens reagent, care must be taken not to have excess ammonia which makes the test much less sensi- tive. It is known that ammonia readily reacts with aldehydes to give a variety of addition products. These addition reactions would reduce the amount of free aldehyde available for reac-
What color is a positive Tollens test?
The formation of a dark grey precipitate or silver mirror on the bottom and sides of the test tube indicates a positive result, which means that the given sample contains reducing sugars/ aldoses. What is Tollens test used for?