Does coupling with ATP hydrolysis affect delta G?
The hydrolysis of ATP is coupled with many biological reactions changing the value of their delta G into a negative value.
The ΔG of ATP hydrolysis in a test tube under standard conditions is -7.3 kcal/mol.
The ΔG for the reaction A + B = C under the same conditions is +8.2 kcal/mol.
Why does the hydrolysis of ATP have a negative ∆ G value?
The negative value of delta G in the hydrolysis of ATP is that since the products of this hydrolysis process are stable and have lower energy than ATP.
How much energy is released by ATP hydrolysis?
Energy Output in ATP Hydrolysis
The hydrolysis of 1M of ATP into ADP and inorganic phosphate, releases −7.3 kcal/mol of energy.
The energy released in the living cell is almost double the value in standard conditions, it is equal to −14 kcal/mol.
Overview
ATP structure, ATP hydrolysis to ADP, and reaction coupling. Introduction A cell can be thought of as a small, bustling town. Carrier proteins move substances into and out of the cell, motor proteins carry cargoes along microtubule tracks, and metabolic enzymes busily break down and build up macromolecules. Even if they would not be energetically favorable (energy-releasing, or exergonic) in isolation, these processes will continue merrily along if there is energy available to power them (much as business will continue to be done in a town as long as there is money flowing in). However, if the energy runs out, the reactions will grind to a halt, and the cell will begin to die. Energetically unfavorable reactions are “paid for” by linked, energetically favorable reactions that release energy. Often, the "payment" reaction involves one particular small molecule: adenosine triphosphate, or ATP. ATP structure and hydrolysis khanacademy.org
Introduction
A cell can be thought of as a small, bustling town. Carrier proteins move substances into and out of the cell, motor proteins carry cargoes along microtubule tracks, and metabolic enzymes busily break down and build up macromolecules. Even if they would not be energetically favorable (energy-releasing, or exergonic) in isolation, these processes will continue merrily along if there is energy available to power them (much as business will continue to be done in a town as long as there is money flowing in). However, if the energy runs out, the reactions will grind to a halt, and the cell will begin to die. khanacademy.org
ATP structure and hydrolysis
Adenosine triphosphate, or ATP, is a small, relatively simple molecule. It can be thought of as the main energy currency of cells, much as money is the main economic currency of human societies. The energy released by hydrolysis (breakdown) of ATP is used to power many energy-requiring cellular reactions. Structurally, ATP is an RNA nucleotide that bears a chain of three phosphates. At the center of the molecule lies a five-carbon sugar, ribose, which is attached to the nitrogenous base adenine and to the chain of three phosphates. khanacademy.org
Hydrolysis of ATP
Why are the phosphoanhydride bonds considered high-energy? All this really means is that an appreciable amount of energy is released when one of these bonds is broken in a hydrolysis (water-mediated breakdown) reaction. ATP is hydrolyzed to ADP in the following reaction: ATP+H2O⇋ADP+Pi+energy Note: Pi just stands for an inorganic phosphate group (PO43−) . Like most chemical reactions, the hydrolysis of ATP to ADP is reversible. The reverse reaction, which regenerates ATP from ADP and Pi , requires energy. Regeneration of ATP is important because cells tend to use up (hydrolyze) ATP molecules very quickly and rely on replacement ATP being constantly produced1 . You can think of ATP and ADP as being sort of like the charged and uncharged forms of a rechargeable battery (as shown above). ATP, the charged battery, has energy that can be used to power cellular reactions. Once the energy has been used up, the uncharged battery (ADP) must be recharged before it can again be used as a power source. The ATP regeneration reaction is just the reverse of the hydrolysis reaction: energy+ADP+Pi⇋ATP+H2O khanacademy.org
Reaction coupling
How is the energy released by ATP hydrolysis used to power other reactions in a cell? In most cases, cells use a strategy called reaction coupling, in which an energetically favorable reaction (like ATP hydrolysis) is directly linked with an energetically unfavorable (endergonic) reaction. The linking often happens through a shared intermediate, meaning that a product of one reaction is “picked up” and used as a reactant in the second reaction. When two reactions are coupled, they can be added together to give an overall reaction, and the ΔG of this reaction will be the sum of the ΔG values of the individual reactions. As long as the overall ΔG is negative, both reactions can take place. Even a very endergonic reaction can occur if it is paired with a very exergonic one (such as hydrolysis of ATP). For instance, we can add up a pair of generic reactions coupled by a shared intermediate, B, as follows2 : A⇋BΔG=X+B⇋C+DΔG=YA⇋C+DΔG=X+Y You might notice that the intermediate, B, doesn't appear in the overall coupled reaction. This is because it appears as a both a product and a reactant, so two Bs cancel each other out when the reactions are added. khanacademy.org
ATP in reaction coupling
When reaction coupling involves ATP, the shared intermediate is often a phosphorylated molecule (a molecule to which one of the phosphate groups of ATP has been attached). As an example of how this works, let’s look at the formation of sucrose, or table sugar, from glucose and fructose3,4 . khanacademy.org
Case study: Let's make sucrose
The formation of sucrose requires an input of energy: its ΔG is about +27 kJ/mol (under standard conditions). ATP hydrolysis has a ΔG around −30 kJ/mol under standard conditions, so it can release enough energy to “pay” for the synthesis of a sucrose molecule: glucose +fructose⇋sucroseΔG=+27 kJ/molATP+H2O⇋ADP+PiΔG=−30 kJ/molglucose +fructose+ATP⇋sucrose+ADP+PiΔG=−3kJ/mol How is the energy released in ATP hydrolysis channeled into the production of a sucrose molecule? As it turns out, there are actually two reactions that take place, not just one big reaction, and the product of the first reaction acts as a reactant for the second. •In the first reaction, a phosphate group is transferred from ATP to glucose, forming a phosphorylated glucose intermediate (glucose-P). This is an energetically favorable (energy-releasing) reaction because ATP is so unstable, i.e., really "wants" to lose its phosphate group. •In the second reaction, the glucose-P intermediate reacts with fructose to form sucrose. Because glucose-P is relatively unstable (thanks to its attached phosphate group), this reaction also releases energy and is spontaneous. This example shows how reaction coupling involving ATP can work through phosphorylation, breaking a reaction down into two energetically favored steps connected by a phosphorylated (phosphate-bearing) intermediate. This strategy is used in many metabolic pathways in the cell, providing a way for the energy released by converting ATP to ADP to drive other reactions forward. khanacademy.org
Different types of reaction coupling in the cell
The example above shows how ATP hydrolysis can be coupled to a biosynthetic reaction. However, ATP hydrolysis can also be coupled to other classes of cellular reactions, such as the shape changes of proteins that transport other molecules into or out of the cell. khanacademy.org
Case study: Sodium-potassium pump
It’s energetically unfavorable to move sodium (Na+ ) out of, or potassium (K+ ) into, a typical cell, because this movement is against the concentration gradients of the ions. ATP provides energy for the transport of sodium and potassium by way of a membrane-embedded protein called the sodium-potassium pump (Na+/K+ pump). In this process, ATP transfers one of its phosphate groups to the pump protein, forming ADP and a phosphorylated “intermediate” form of the pump. The phosphorylated pump is unstable in its original conformation (facing the inside of the cell), so it becomes more stable by changing shape, opening towards the outside of the cell and releasing sodium ions outside. When extracellular potassium ions bind to the phosphorylated pump, they trigger the removal of the phosphate group, making the protein unstable in its outward-facing form. The protein will then become more stable by returning to its original shape, releasing the potassium ions inside the cell. Although this example involves chemical gradients and protein transporters, the basic principle is similar to the sucrose example above. ATP hydrolysis is coupled to a work-requiring (energetically unfavorable) process through formation of an unstable, phosphorylated intermediate, allowing the process to take place in a series of steps that are each energetically favorable. [Attribution and references] khanacademy.org
Want to join the conversation?
Log in khanacademy.org

ATP hydrolysis mechanism Energy and enzymes Biology Khan Academy

ATP hydrolysis: Gibbs free energy Biomolecules MCAT Khan Academy

Mechanism of ATP Hydrolysis
|
Free Energy of ATP hydrolysis manipulates the cellular calcium
show that the available Gibbs free energy of ATP hydrolysis (?G) which measures the “distance” of a reaction to its equilibrium state |
|
Calculations-in-bioenergetics.pdf
?G´ free enthalpy change at pH 7 (J mol –1) What is the value of the equilibrium constant of ATP hydrolysis at 37 0C. [ADP] [Pi]. Keq =. |
|
Untitled
the transformed free energy G' is the criterion for equi- librium.) By convention when HO |
|
Bio UN2005/F2401x 2017 Lec.8 L. Chasin October 3 2017 Free
IF Delta G IS 0 THEN THE REACTION WILL BE AT EQUILIBRIUM: NOT like ATP hydrolysis that release at least 7 kcal/mole |
|
Glycolysis I Highlights
energy-coupled reaction and the Delta G zero prime is strongly negative thanks to the ATP hydrolysis. 4. Hexokinase changes shape as it binds to glucose. |
|
Solutions to 7.014 Problem Set 2
?G o = ?3.4 kcal mol a) What two functions does ATP serve in this coupled reaction? The hydrolysis of ATP provides the energy to drive this coupled |
|
Bioenergetics Free Energy Change
The free energy change (?G) of a chemical process is a Biochemical reactions are usually coupled to the hydrolysis of ATP: ATP ??ADP + Pi ?Go'= -7.3 ... |
|
Fall 2009 333 HW 3KEY
23 Kas 2009 know how much ATP (in moles) is generated from the complete oxidation ... What is the ?G?' of ATP hydrolysis according to these data and is ... |
|
Use of cardiac magnetic resonance to detect changes in metabolism
ATP hydrolysis (?G'ATP hydrolysis or simply ?G) and provides Figure 3 Conception of Delta-G-ATP as a water cylinder exerting pressure. |
|
Class examples relating to biology and medicine - MIT
The hydrolysis of ATP (ATP → ADP), a spontaneous process, can be coupled to a non- spontaneous reaction to drive the non-spontaneous reaction forward ATP |
|
Chem331 Lect 17 Thermodynamics - University of San Diego Home
Example: Hydrolysis of Phosphoenolpyruvate (PEP) to drive ATP synthesis ADP + Pi → ATP + H2O; ΔG°' = +55 kJ/mol Need to couple it to another, very |
|
Metabolic Energy - Oregon State University
Creatine Kinase Creatine + ATP Creatine Phosphate + ADP ΔG°' Values of Hydrolysis (kJ/mol) Oh Delta G - the change in Gibbs free energy Can tell |
|
Bioenergetics Free Energy Change - CSUN
∆Go' for ATP hydrolysis is more negative than most biochemical reactions and than hydrolysis of phophorylated comounds such as Glc-6-PO4 or Glycerol-3- PO4 |
|
Calculations in Bioenergetics
What is the value of the equilibrium constant of ATP hydrolysis at 37 0C [ADP] [Pi ] Calculate the free enthalpy change ∆G at the ratio ATP/ADP = 100:1 |
|
The Mechanism of Hydrolysis Reaction of Adenosine Triphosphate
29 jui 2017 · generation of bio-energy released in ATP hydrolysis and its features Obviously, these and beta and delta subunits during catalysis |
|
QUIZ 1 The hydrolysis reaction, ATP + H2O → ADP + phosphate
The hydrolysis reaction, ATP + H2O → ADP + phosphate, has a standard free energy, AGoʼ, of -30 5 kJ/mol (a) or (c) Show the formula for the equilibrium |
|
ATP synthase
Hydrolysis Synthesis ∆G = ∆G0 + ln ([ADP][P i]/[ATP]) = -13 7 kcal/mol ~1/500 ATP hydrolysis shifts the equilibria of coupled reactions Coupled reaction: |
|
[PDF] Bioenergetics Free Energy Change - CSUNedu
The free energy change (∆G) of a chemical process is a measure of the Biochemical reactions are usually coupled to the hydrolysis of ATP ATP ←→ ADP + |
|
[PDF] Metabolic Energy - Oregon State University
Creatine Kinase Creatine + ATP Creatine Phosphate + ADP ΔG°' Values of Hydrolysis (kJ mol) Oh Delta G the change in Gibbs free energy Can tell |
|
[PDF] QUIZ 1 The hydrolysis reaction, ATP + H2O → ADP + phosphate
The hydrolysis reaction, ATP + H2O → ADP + phosphate, has a standard free energy, AGoʼ, of 305 kJ mol (a) or (c) Show the formula for the equilibrium |
|
[PDF] Class examples relating to biology and medicine - MIT
Recall from Lecture 17 that the ∆H° of ATP hydrolysis is 24 kJ mol of ATP + 2H2O (l) The hydrolysis of ATP (ATP → ADP), a spontaneous process, can be coupled to a non 3 H bonds between C and G 2 H bonds between A and T |
|
[PDF] [ADP] [ATP] + [ADP] - WordPresscom
which ATP is synthesized 1) Calculate the ∆G' for step 7 of glycolysis Hydrolysis of 1,3 BPG is coupled to the synthesis of ATP The oxidation energy of a |
|
[PDF] ATP synthase
ATP hydrolysis shifts the equilibria of coupled reactions Coupled reaction A ↔ B ∆ G°' = + 40 kcal mol ATP + H2O ↔ ADP + Pi + H+ ∆ G°' = 73 kcal mol |
|
[PDF] Chem 301
If the stoichiometry of transport were 1 mol of glucose transported per mole of ATP hydrolyzed, what would be the maximum concentration gradient of glucose |
|
Mechanochemical coupling of the motion of molecular - Cell Press
number of ATP molecules hydrolyzed and the turnover of the output reaction Recent experiments a Dirac delta function at the minimum of the potential Pu and PB define the 37 405 425 Peskin, C S, B Ermentrout, and G Oster 1994 |
|
[PDF] 61 Energy and Metabolism - The Expert TA
Every chemical reaction involves a change in free energy, called delta G (∆G) Cells couple the ATP hydrolysis' exergonic reaction allowing them to proceed |
- phosphocreatine + adp → creatine + atp delta g
- calculate the heat produced by hydrolysis of 65 kg of atp
- direct vs indirect coupling biochemistry
- atp hydrolysis free energy
- delta g metabolism
- calculate delta g for atp hydrolysis
- atp to adp
- the energy released by the hydrolysis of atp is
- does coupling with atp hydrolysis affect delta g
![Energy in breaking bonds [ATP and ADP]? Energy in breaking bonds [ATP and ADP]?](https://cdn.kastatic.org/ka-perseus-images/53f152422af5dca84530fb7d1187295c7d9d5526.png)
Energy in breaking bonds [ATP and ADP]?
Source:https://cdn.kastatic.org/ka-perseus-images/53f152422af5dca84530fb7d1187295c7d9d5526.png
ATP cycle and reaction coupling
Source: Energy (article)
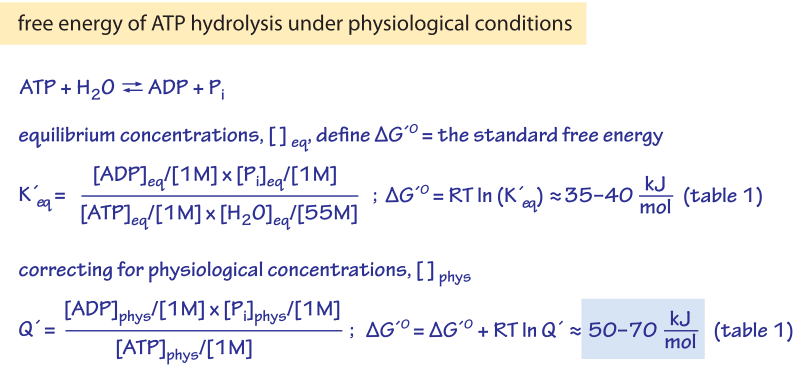
PDF) Thermodynamics of the hydrolysis of sucrose
Source:http://book.bionumbers.org/wp-content/uploads/2014/08/335-f1-ATPHydrolysisCalc-1.png

How much energy is released in ATP hydrolysis?
Source:https://i.ytimg.com/vi/BzVZO6abaMs/maxresdefault.jpg
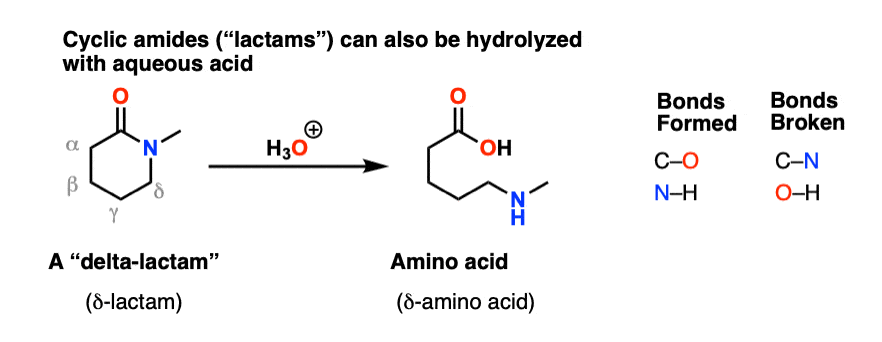
Hydrolysis of sucrose gives `Sucrose +H_(2)OhArrGlucose + Fructose
Source:https://cdn.masterorganicchemistry.com/wp-content/uploads/2019/10/3-cyclic-amide-is-called-a-lactam-this-is-an-example-of-a-delta-lactam-six-membered-lactam.gif

Amide Hydrolysis Using Acid Or Base – Master Organic Chemistry
Source:https://i1.rgstatic.net/publication/234004224_Spontaneous_hydrolysis_of_ethyl_formate_Isobaric_activation_parameters/links/5ee7815b299bf1faac560897/largepreview.png
atp pc system duration
Contribution of energy systems during a Wingate power test
- atp-pc system sporting examples
- Lactic acid system duration
- Aerobic system duration
- Glycolytic system
- atp-pc system fuel
- Energy systems
- ATP-PCr system
- Anaerobic energy system
- atp pc system time
- atp-pc system recovery time
atp pc system sporting examples
[PDF] Energy systems and physical activity - bss12pe
- Glycolytic systemAerobic energy systemLactic acid system sporting examples
- Energy systems
- Aerobic system
- ATP-PC system duration
- ATP-PCr system
- Energy systems in sport
- atp-pc system sporting examples
- atp-pc system sports
- atp pc energy system sporting examples
- atp-pc system btec sport
- atp-pc energy system sports
- atp-pc system use in sport
atrial septal defect adalah pdf
[PDF] Pathophysiology and Therapy for Atrial Septal Defects - Cardiac
- atrial septal defect ppt
- ventricular septal defect pdf
- atrial septal defect pathophysiology
- atrial septal defect types
- atrial septal defect adalah pdf
- atrial septal defect adalah
- atrial septal defect secundum adalah
- atrial septal defect (asd) adalah
- diagnosis atrial septal defect adalah
atrial septal defect echo ppt
[PDF] Indications and Evaluation for ASD Closure - Cardiac Interventions
- atrial septal defect echo findings
- atrial septal defect echo ppt
- atrial septal defect echo
- atrial septal defect echo criteria
- primum atrial septal defect echo
- atrial septal defect size echo
- sinus venosus atrial septal defect echo
- ostium primum atrial septal defect echo