|
CE 123
CE 123 A Guide to Understanding European T echnical Regulations and CE standards standards testing notified body services etc This will be for |
What does CE mean on medical devices?
Those products are subject to CE marking for medical devices.
Obtaining the CE, or Conformité Européenne, medical device marking, means that your product meets all applicable health, safety, and environmental regulations in the EU, and you are legally able to sell it.What is the CE mark number 0123?
CE mark with four-digital identification number of NB A CE marking followed by a four-digit identification number indicates that a Notified Body (NB) was involved in conformity assessment.
CE 0123 shows that TÜV SÜD was the Notified Body involved in conformity assessment.The CE marking (an acronym for the French “Conformite Europeenne”) certifies that a product has met EU health, safety, and environmental requirements, which ensure consumer safety.
What is a CE notified body?
A notified body is an organisation designated by an EU Member State (or by other countries under specific agreements) to assess the conformity of certain products before being placed on the market.
|
LIST OF BODIES NOTIFIED UNDER DIRECTIVE : 93/42/EEC
2006/42/EC on machinery. Creation Date : 31/07/2022. LIST OF BODIES NOTIFIED UNDER DIRECTIVE : 93/42/EEC Medical devices. Name and address of the notified. |
|
CE 123 . . . A Guide to Understanding European T echnical
This is a preview of "CE 123.". CHAPTER 6 – THE NOTIFIED BODIES ... development organizations notified bodies and surveillance authorities. |
|
REGULATION (EU) 2017/ 745 OF THE EUROPEAN PARLIAMENT
5 mag 2017 (42) 'notified body' means a conformity assessment body designated in accordance with this Regulation;. (43) 'CE marking of conformity' or ... |
|
LIST OF BODIES NOTIFIED UNDER DIRECTIVE : 97/23/EC
20 lug 2016 0026. Pressure equipment. Internal manufacturing checks with monitoring of the final assessment. EC type-examination. Conformity to type. |
|
MDCG 2021-1 Rev.1 - Guidance on harmonised administrative
26 mag 2021 of relevant economic operators (actor registration) devices and systems and procedure packs (UDI) |
|
REGULATION (EU) 2017/ 746 OF THE EUROPEAN PARLIAMENT
5 mag 2017 (34) 'notified body' means a conformity assessment body designated in accordance with this Regulation;. (35) 'CE marking of conformity' or ... |
|
Draft Guideline Drug Device Combinations - for adoption - 20190508
29 mag 2019 integral non-integral |
|
MDCG 2021-25
19 ott 2021 PSUR and notified bodies' appropriate surveillance. MDCG members from BE DE |
|
LIST OF BODIES NOTIFIED UNDER DIRECTIVE : Regulation (EU
LIST OF BODIES NOTIFIED UNDER DIRECTIVE : Regulation (EU) No 305/2011 - Construction products. Name and address of the notified bodies. Iden. number. |
|
LIST OF BODIES NOTIFIED UNDER DIRECTIVE : 2006/42/EC
0026. 1.1. Circular saws (single- or multi-blade) - sawing machinery with fixed blade(s) during cutting having a fixed bed or support with manual feed of |
|
LIST OF BODIES NOTIFIED UNDER DIRECTIVE : 93/42/EEC
LIST OF BODIES NOTIFIED UNDER DIRECTIVE : 93/42/EEC Medical devices Annexes or articles of the directives Limitations (English only) 123 / 320 |
|
Text CE 19 Anyco Ltd, PO Box 123, Leeds UK Product A1234
CE 19 Anyco Ltd, PO Box 123, Leeds UK Product A1234 – DoP No 5678 EN 123-5: 2009 Notified Body No 2511 Internal external use in walls partitions |
|
40bbbcf7-6f92-4169-a2d6-8e0892108f1fpdf
19 Anyco Ltd, PO Box 123, Leeds, UK Product A1234 - DoP No 5678 --- EN123-5: 2009 -- Notified Body No 2511 Internal external use in walls partitions |
|
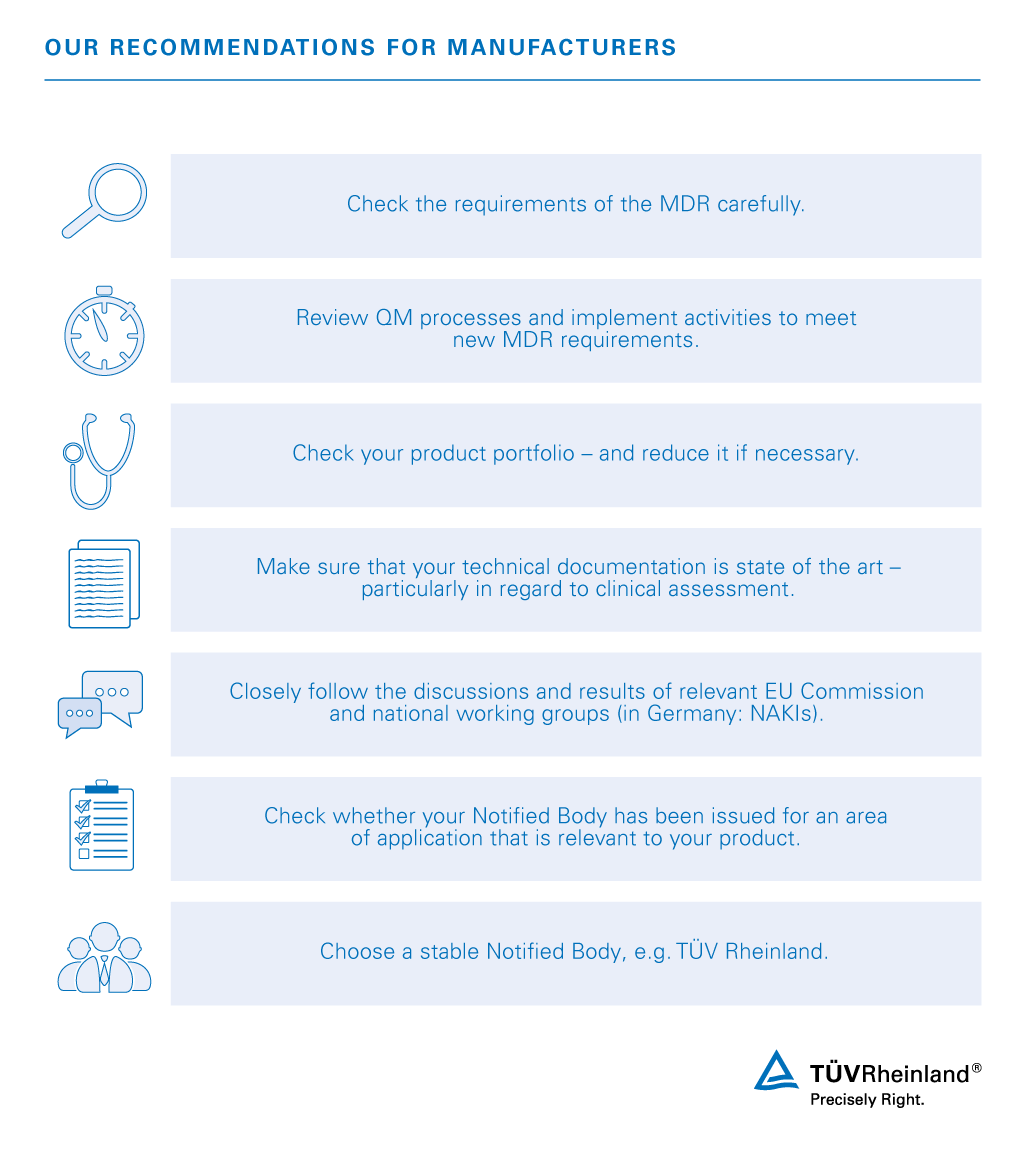
EU MDR Overview, Compliance, and Implementation Strategy
should get them certified by their Notified Body before May 26, 2020 The new EU Medical Device Regulation (MDR) includes 123 Articles and 17 Annexes |
|
The Notified Body Point of View European - Healthcapital
20 jui 2018 · Challenges for the Notified Bodies and Economic Operators • Important Transition Important Transition Provisions (MDR - Article 120 + 123) |
|
MDR Compliance Postponed Until May 2021 - NSF International
26 mai 2020 · notified bodies that were no longer able to perform audits, to from articles 123(f ) and (g) are not affected either The UDI carrier will have to be |
|
[PDF] Notified Bodies and the CE certification Process for - CASCADE
Mar 27, 2014 · The role of Notified Bodies in Medical Device development 1 Prologue Regulatory framework 2 What is a Notified Body 3 CE marking |
|
[PDF] Frequently asked questions - BSI
Certificates to the MDR can be issued from a designated Notified Body after MDR entry into force, and have transition requirements of Article 120 and Article 123, or if there will our trusted QMS certification services, CE Marking or global |
|
[PDF] Technical Documentation and Medical Device Regulation - BSI
or renew a CE certificate or to issue a Declaration of Conformity (DoC), their technical of Notified Bodies Medical Devices (NB MED), on council Directives 90 385 EEC, transition periods for this requirement are determined in Article 123(f) |
|
[PDF] The Regulation of Medical Device Apps - West of England Academic
The manufacturer of the device is free to choose any Notified Body even those that are not based in their own member state to perform the CE Marking assessment |
|
[PDF] ce-marking of construction products - step by step - MGIPU-a
When you, as the manufacturer, affix the CE marking to a product it means that a number of Notified Bodies to carry out tasks related to it AnyProduct 123 |
|
[PDF] on the implementation of EU product rules - TAYSAD
CE marking requirements, notified bodies, market surveillance, accreditation, for the purpose and does not go beyond what is necessary to achieve it123 35 |
|
[PDF] RIVM rapport 318902013 Disinfectants for medical devices - VGT
manufacturers of disinfectants and the notified bodies Special Identification of the notified bodies that were involved in the CE conformity procedure followed by the Else Kröner Str 1, 61346 Bad Homburg vd H, Duitsland 123 Micro 10+ |
- CE 0123 Notified Body
- CE 2797 Notified Body
- Nando list of Notified bodies
- Ce mark and identification number of notified body
- [PDF] ce-marking of construction products - step by step - MGIPU-ahttps://mgipu.gov.hr › dokumenti › Graditeljstvo › GradevniProizvodi
- When you
- as the manufacturer
- affix the CE marking to a product it means that ... a number of Notified Bodies to carry out tasks related to it. ... AnyProduct-123.[PDF] The Notified Body Point of View European ... - Healthcapitalhttps://www.healthcapital.de › documents › Publikationen › Zietsch
- Jun 20
- 2018 · Challenges for the Notified Bodies and Economic Operators. • Important Transition ... Important Transition Provisions (MDR - Article 120 + 123). • 05.05. 2017 Publication ... E-Mail: mdc@mdc-ce.de mdc medical device ...[PDF] on the implementation of EU product rules - TAYSADhttps://www.taysad.org.tr › 26-03-2014-11-52-Blue-Guide-2014
- CE marking requirements
- notified bodies
- market surveillance
- accreditation
- ... for the purpose and does not go beyond what is necessary to achieve it123. 3.5.[PDF] RIVM rapport 318902013 Disinfectants for medical devices - VGTwww.vgt.nl › downloads › pdf › rapport_rivm_2003
- manufacturers of disinfectants and the notified bodies. Special ... Identification of the notified bodies that were involved in the CE conformity procedure followed by the ... Else-Kröner-Str. 1
- . 61346 Bad Homburg vd H
- . Duitsland. 123. Micro-10+.Related searchesCE 1293 notified Body
- List of notified bodies under MDR
- CE 1639 Notified Body
- SUNGO Notified body
- CE notified body list in India
- CE certification Body
- Notified Bodies in france
- Ce notified body list ppe

COVID-19 : suspicious certificates for PPE
Source:https://img.yumpu.com/44408735/1/500x640/certificate-modulo-b-pdf-albatross-srl.jpg

certificate modulo B pdf - albatross srl
Source:https://0.academia-photos.com/attachment_thumbnails/37729097/mini_magick20190228-14879-iv5aev.png?1551384210

PDF) Best Practices to Prepare for and Manage Compliance Audits by
Source:https://www.eu-esf.org/images/COVID-19/example_NTC.png
COVID-19 : suspicious certificates for PPE
Source:https://www.tuv.com/content-media-files/master-content/services/products/p05-medical/1665-tuv-rheinland-eu-medical-device-regulation-mdr-2017-745/tuv-rheinland-medical-device-regulation-visual-1-en.png
EU Medical Device Regulation MDR 2017/745
Source: WO

Notified body - Wikipedia
Source:https://www.eu-esf.org/images/COVID-19/example_VIC.jpg
ce 123 reddit
[PDF] Approved H-1B Petitions - USCIS
ce 1234
Codice DB1117 DD 19 novembre 2014, n 1005 Regolamento CE
- ce 1234/07
- 12345 ce n'est pas 6
- reglamento ce 1234 de 2007 consolidado
- reg ce 1234/07
- regolamento ce 1234/07
- règlement ce 1234 de 2007
- reg ce 1234 del 2007
- regolamento ce 1234 del 2007
ce 1282 fda
[PDF] dossier-certif-masques-ts-minpdf - Toner Services
ce 1282 mascherine
[PDF] Sicurezza e protezione sul lavoro >> scarica il PDF - Frigerio SA
- N95 mask
- FDA KN95
- KN95 mask
- BYD KN95 Particulate Respirator
- [PDF] Studio clinico N95 vs Mascherine chirurgiche IIR Il paper in ... - Medifixwww.medifixsanitaria.it › PDF › N95 vs Mascherine Chirurgiche IIR
- Piccola premessa: • le maschere N95 sono classificate secondo lo standard americano gestito dal NIOSH ... respiratori N95 o maschere mediche. ... 1282 ( 52.4).[PDF] COVID19www.crosa.it › uploads › COVID19-European-Safety-Federation
- Apr 22
- 2020 · CE dei DPI (comprese maschere FFP2 / FFP3 e protezione degli occhi)
- ... identificazione dell'organismo notificato ECM 1282 accanto a CE ...[PDF] Oggetto: Nuova normativa EN 149:2001+A1:2009extranet.3m.eu › mysafety › it-it › items › download
- La rispondenza di un qualunque tipo di dispositivo ai requisiti della Direttiva DPI è in parte evidenziata testando i requisiti di prestazione del dispositivo stesso ...[PDF] Sicurezza e protezione sul lavoro >> scarica il PDF - Frigerio SAwww.frigerio.ch › images › 2_Media › DPI_catalogo_FRIGERIO
- e a mascherina
- simbolo "occhiali di protezione"
- 4 etichette autocollanti per ... 1282. 129. .0040 40. 1333. 129. .0041 41. 1409. 129. .0042 42. 1478. 129.Related searchesArun KN95
- BYD DG3101
- Powecom KN95
- KN95 FDA approved
- Non NIOSH-approved
- FDA EUA
- FDA approved KN95 masks
- KN95 mask China
- ce 1282 mascherine significato
- ce 1282 mascherina