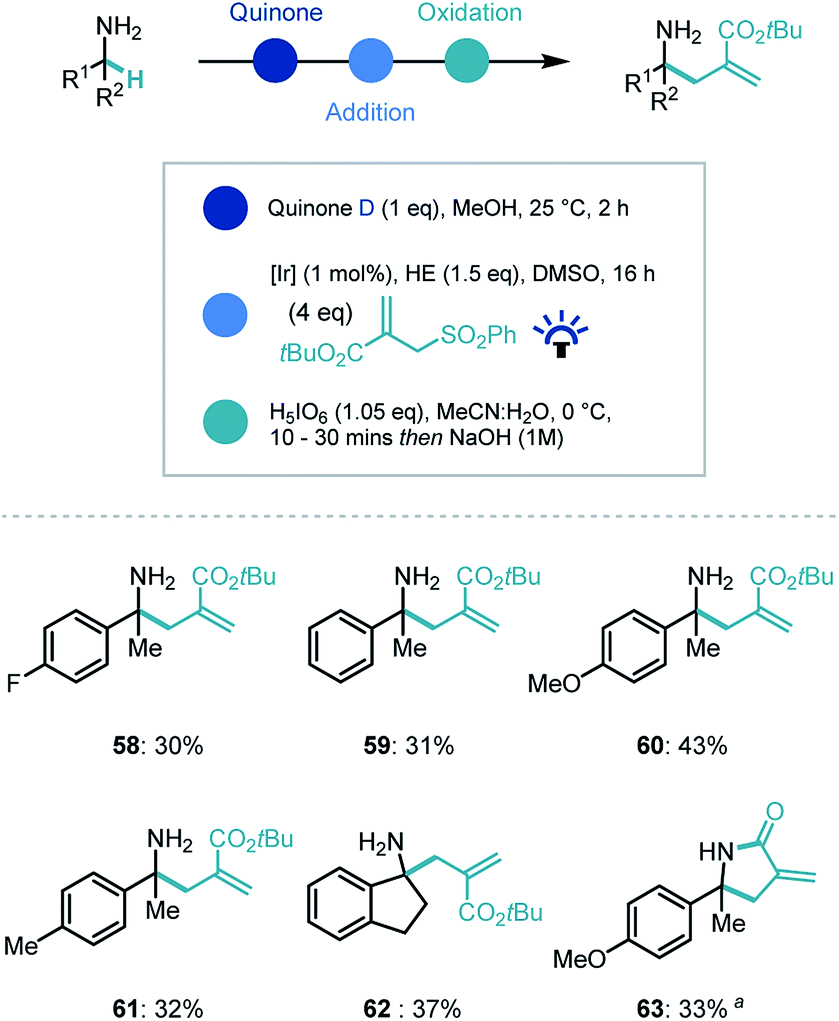
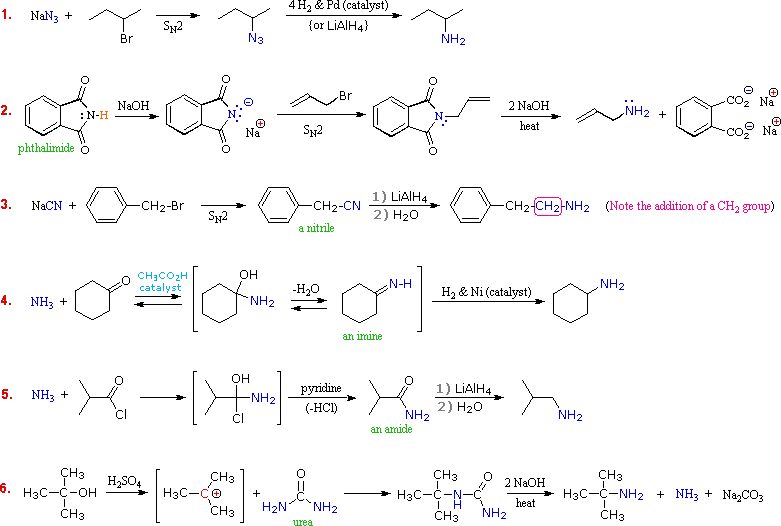
primary amine + naoh
|
Reactions of Amines
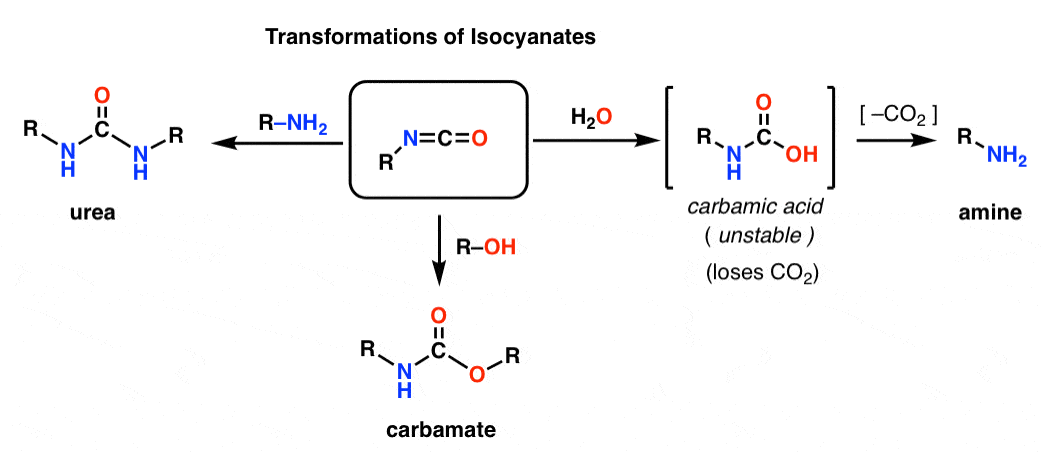
Reactions of Amines. 1. Reaction as a proton base (Section 19-5 and 19-6). R N. H. H. N. H. H. R. H. X. H-X (proton acid). NaOH amine base ammonium salt. |
|
Reactions of Amines
Reactions of Amines. 1. Reaction as a proton base (Section 19-5 and 19-6). R N. H. H. N. H. H. R. H. X. H-X (proton acid). NaOH amine base ammonium salt. |
|
Dissolving Carboxylic Acids and Primary Amines on the Overhead
9 Mar 2010 Carboxylic acids (or primary amines) that are only slightly soluble in water are dissolved by the addition of aqueous NaOH. (or HCl). |
|
Chapter 6 Amines and Amides
products of amide synthesis and hydrolysis reactions. Amines are classified as primary (1°) secondary ... More complex primary amines are named. |
|
Far beyond primary poly(vinylamine)s through free radical
27 Eki 2015 radical copolymerization and amide hydrolysis ... pendant primary amines among all vinyl polymers.1 Excellent water. |
|
Catalysis of the hydrolysis of isopropyl methylphosphonofluoridate
by Primary Amines consistent with general base catalysis as the mechanism of hydrolysis. ... general base catalyzed hydrolysis;9 nucleophilic attack. |
|
EVALUATION OF MANUFACTURING PROCESSES FOR THE
secondary amine/NaOH and primary amine/NaOH were virtually instantaneous. S-triazine herbicides act by inhibiting primary events in photosynthesis in ... |
|
Testsforfunctionalgroups - inorganiccompounds
Distinction between primary secondary and tertiary alcohols added to the compound in the presence of sodium hydroxide solution. |
| Determine the Reactivity of NHS Esters on Biotinylation and |
|
Supported Ni Catalyst for Liquid Phase Hydrogenation of
4 Oca 2018 ?1·min?1 and primary amine selectivity of 94% when NaOH was added ... The formation of the primary amines has to compete with these side ... |
|
Ch 06 Amines and Amides - Angelo State University
• Amines are organic derivatives of ammonia NH3 in which one or more of the three H’s is replaced by a carbon group • Amines are classified as primary (1°) secondary (2°) or tertiary (3°) depending on how many carbon groups are connected to the nitrogen atom RR''N R' 3° Amine RHN H 1° Amine RHN R' 2° Amine HHN H Ammonia |
|
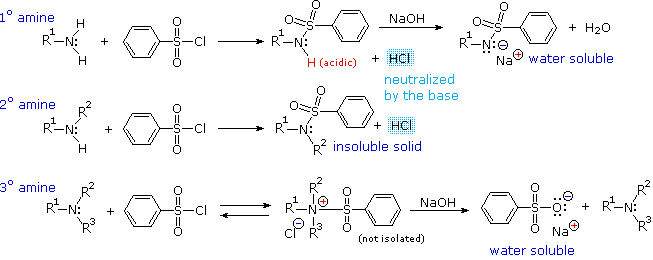
Primary Amine - Chemistry LibreTexts
The Hinsberg Test for Amines (p 330) Read the paragraph on page 330 labeled Amines The following test should distinguish between primary secondary and tertiary amines: Place 100 mg or 8 drops of amine in a 13x100 mm test tube add 6 drops of benzenesulfonyl chloride and 2 mL of methanol Heat the mixture to just below the boiling |
|
Chapter 19: Amines - University of Northern British Columbia
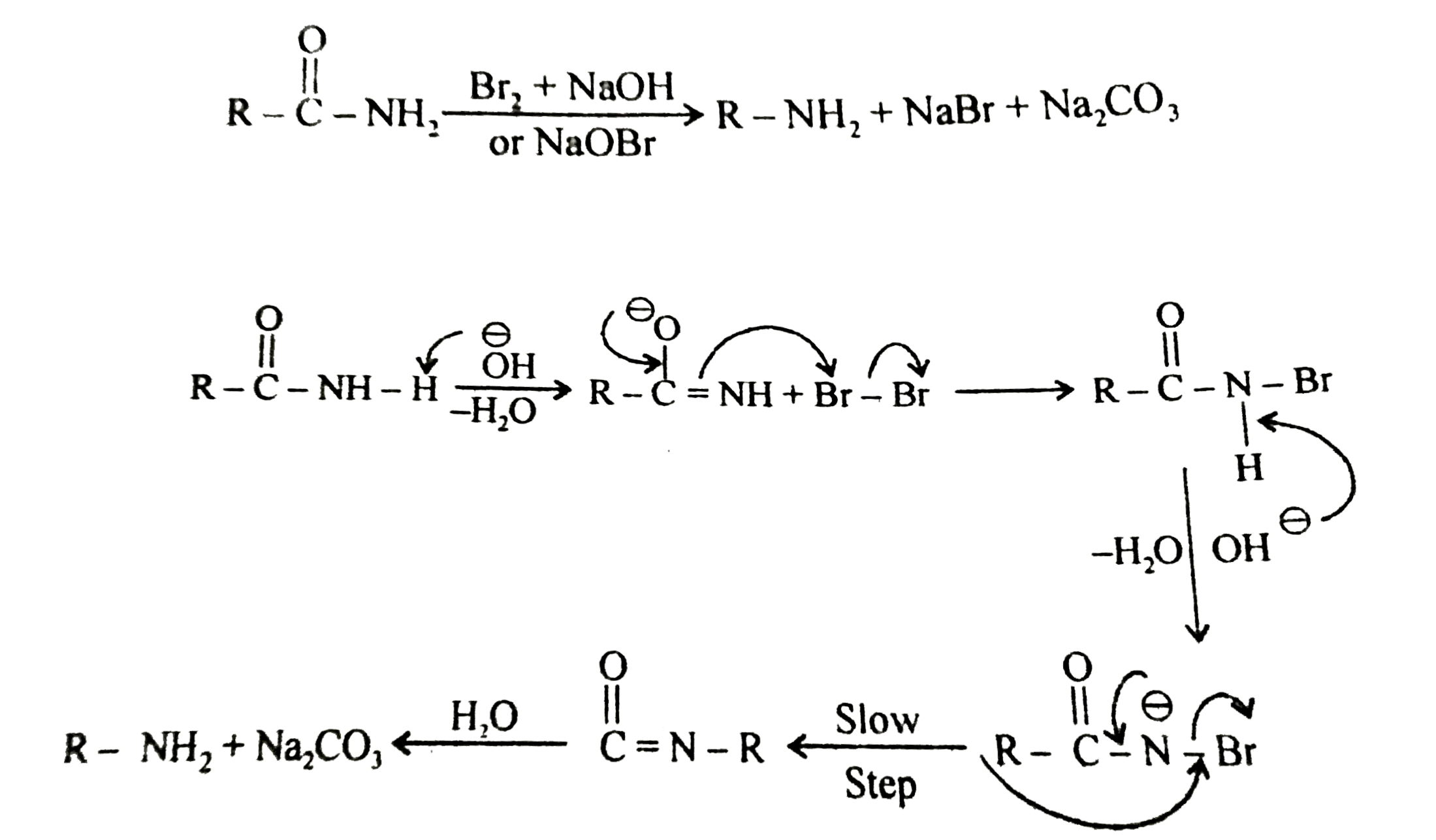
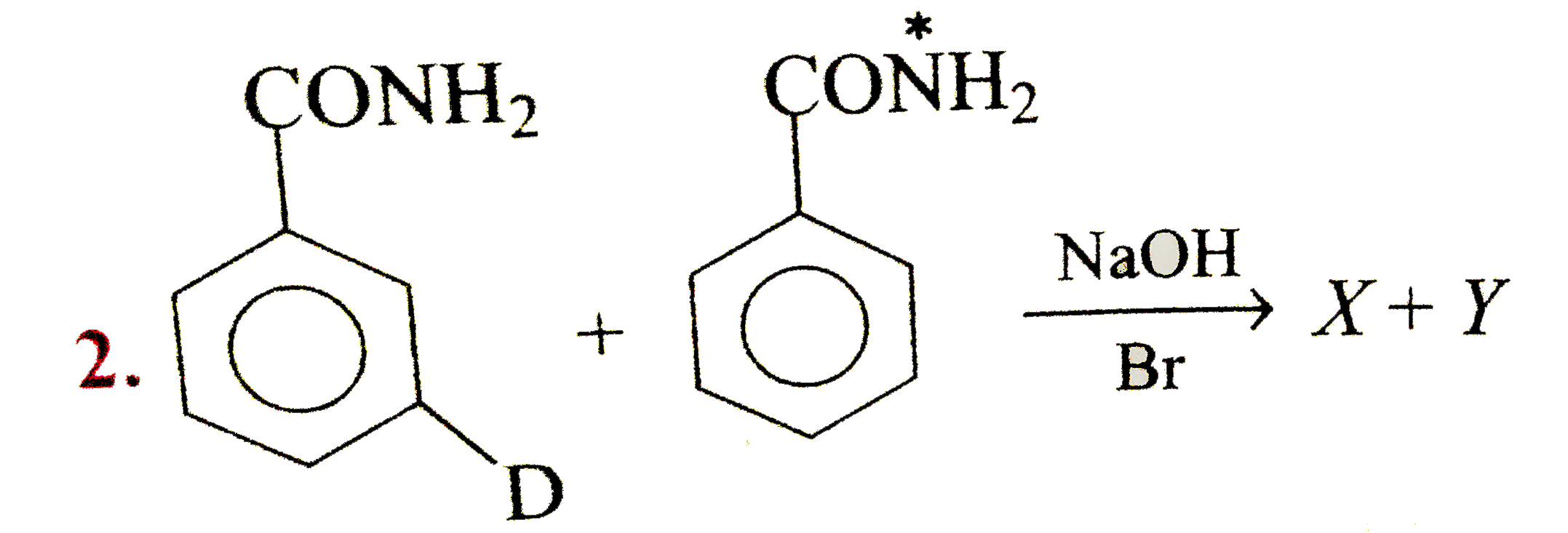
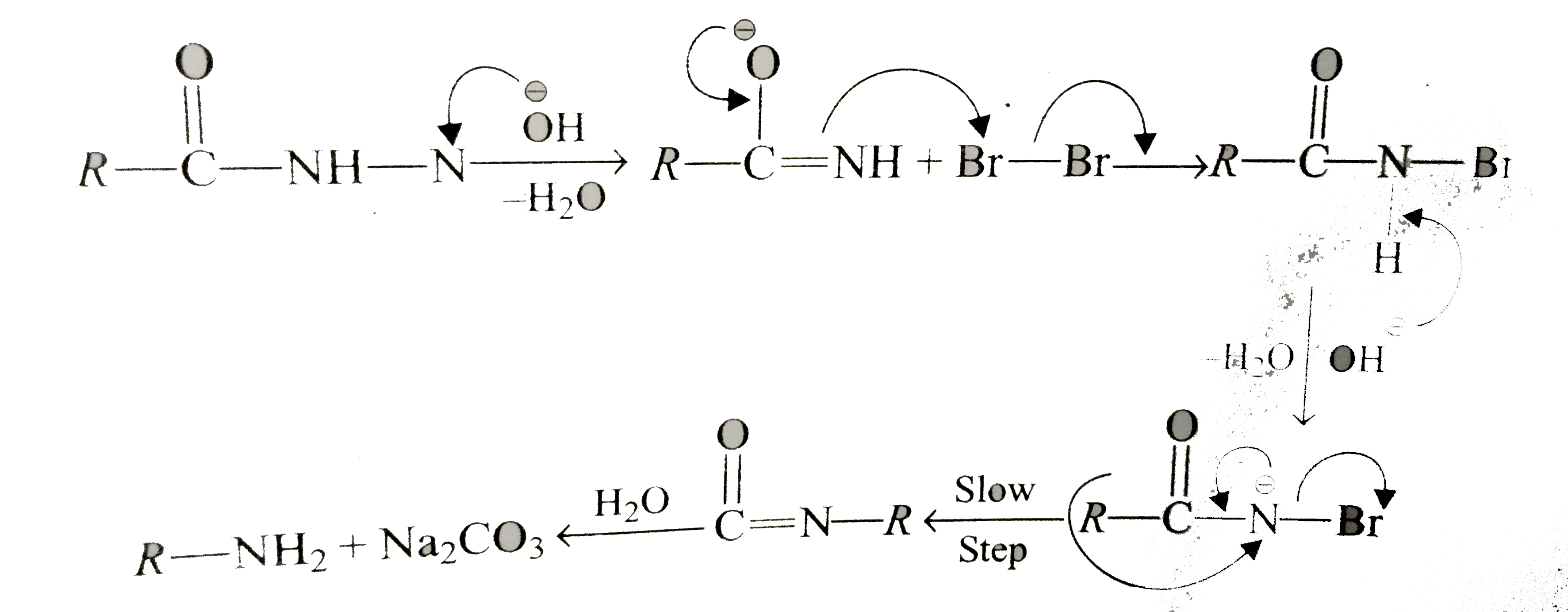
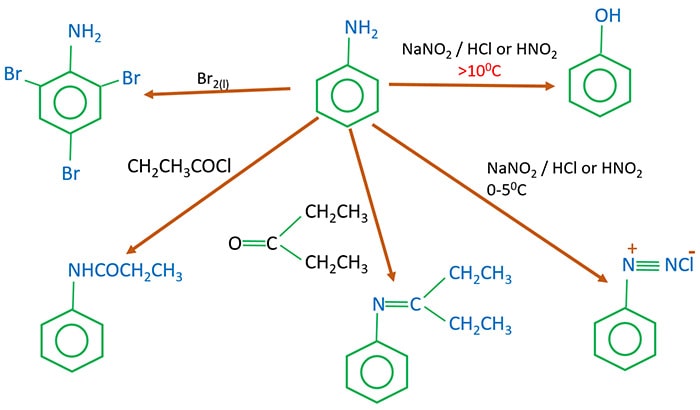
Primary amide react with bromine under basic condition to produce amines via a rearrangement reaction The reaction is limited to primary amide Secondary and tertiary do not give the reaction NH2 O NaOH Br 2/H 2O NH2 + CO2 NH O NaOH Br2/H2O no reaction 414 |
|
Reactions of Amines
ammonia 1¼ amine 1¼ amine 2¼ amine R R 1 O Ketone or aldehyde + H N R 3 R 2 NaBH 3CN cat H+ R R H1 N 2R via R R 1 N R 2¼ amine 3¼ amine 7 Via Amides: (Section 19-20) R N R 1 O R 2 LiAlH 4 R N R 1 R 2 • No mechanism required for the reduction • Access: 1º 2º or 3º Amines • R1 and R2 can be either H or C Thus you can produce |
|
24 Qualitative Organic Analysis – Identification of an Unknown
Most amines are soluble in hydrochloric acid because they form water-soluble ammonium salts NH2+ HClRNH3 Cl Relatively acidic functional groups such as phenols and carboxylic acids are soluble in aqueous NaOH because water-soluble phenoxide and carboxylate salts are formed O +NaOH + H2OROHRONaOH+ NaOH ONa+ H2O |
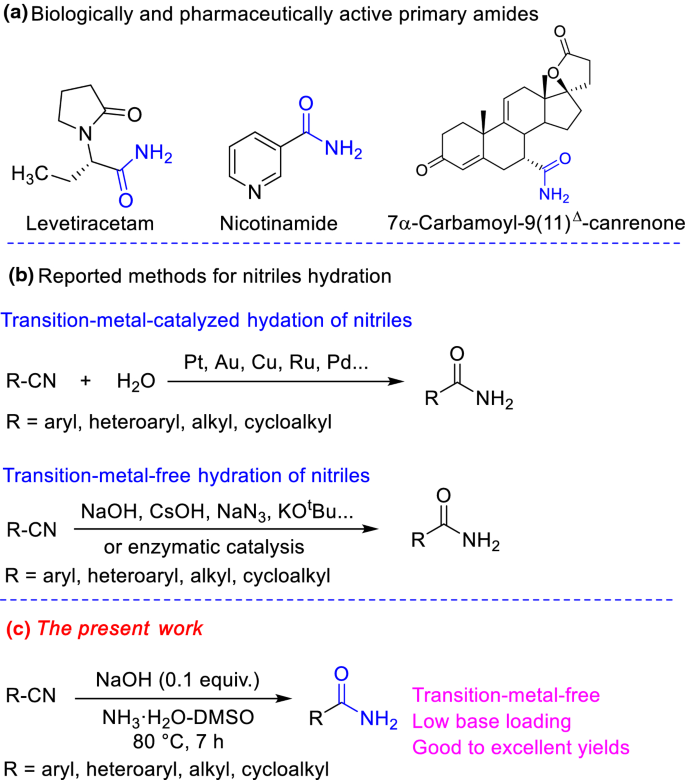
What is a primary (1o) amine?
- A primary (1º) amine is an amine that has the following general structural formula. The NH 2 group in a primary amine molecule is called the primary amine group.
What is the difference between 1° and 2° amines?
- Hydrogen bonding between 1° and 2° amines is not as strong as those found in alcohols or carboxylic acids. 1° and 2° amines have lower boiling points than alcohols of similar molecular weight. 3° amines, since they do not hydrogen bond to each other, have boiling points similar to hydrocarbons of the same molecular weight.
How do alkyl groups affect the basicity of amines?
- Alkyl groups are electron inducing (they seem to act like little electron 'pumps', pushing electrons away from themselves increasing the electron density on the lone pair of the nitrogen. Hence the basicity of amines increases from 1º to 2º to 3º. Aromatic amines have the amine group directly attached to a benzene ring.
What amines react with carbon disulfide and sodium hydroxide?
- Most primary and secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamates. They are used as ligands for chelating metals. Dithiocarbamates readily react with many metal salts such as copper, ferrous, ferric, cobaltous, and nickel salts and are mostly found as octahedral complexes.
|
Reactions of Amines
This involves simple SN2, followed by deprotonation by the excess amine Br excess NH3 NH2 4 Acylation with Acid Chlorides to From Amides: (Section 19- |
|
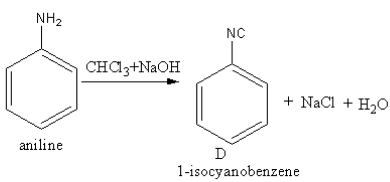
Amines
b) Aromatic Amine: Basic Strength: NH3 > Ar- NH2 > Ar2- NH2 Benzene ring c ) Carbylamine Reaction (Given Only by Primary Amines): C2H5NH2 + CHCl3 + |
|
AMINES
(for aromatic primary amines), and the preparation of salts (picrates, tetraphenylboron salts) in 50 ethanol; 0 5 N NaOH, 2 N acetic acid Procedure: To a few |
|
Chapter 19: Amines
The reaction is limited to primary amide Secondary and tertiary do not give the reaction NH2 O NaOH Br2 /H2 O NH2 + CO2 NH O NaOH Br2 /H2 O |
|
Coupling of substances containing a primary amine to - DiVA
NHS reacts with O-acylisourea, giving a hydrolysis-stable succinimidyl ester, from which a non- dissociated primary amine can attack, resulting in the amide and |
|
NITROGEN CONTAINING COMPOUNDS Amines - Patna Science
good laboratory method for the conversion of an amide to a pure primary amine The amide is warmed with bromine and concentrated aqueous NaOH solution |
|
Experiment 16 Qualitative Analysis of Amines and Amine Unknown
Test in order to discriminate between primary, secondary and tertiary amines NaOH -OH N N OH -OH N N O- Na+ Mechanism Figure 16 6 Azo Dye |