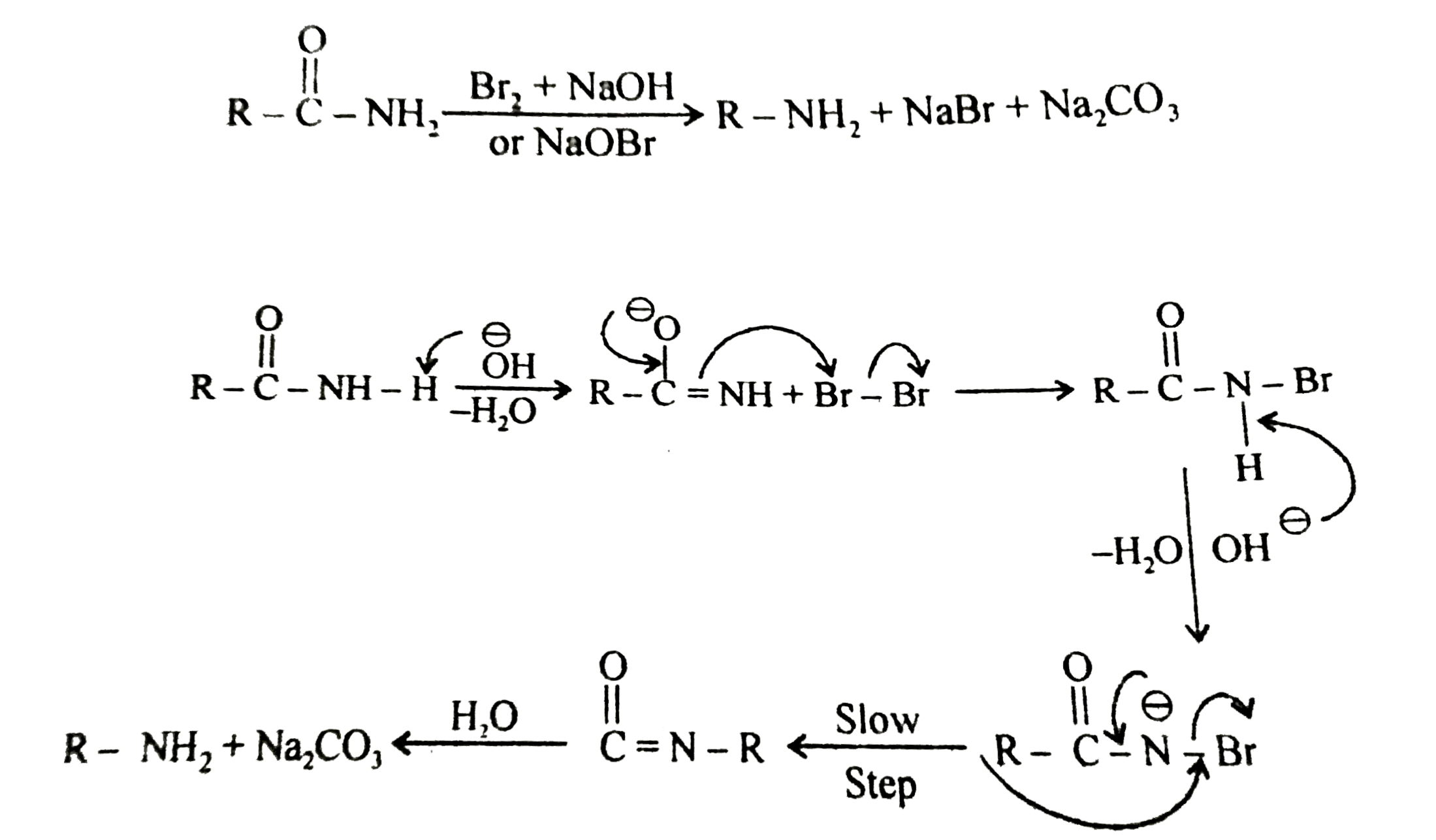
reaction of amide with naoh
|
Chapter 6 Amines and Amides
Learn the major chemical reactions of amines and amides and learn how to predict the products of amide synthesis and hydrolysis reactions. |
|
A mild alkaline hydrolysis of N- and NN-substituted amides and
conditions by the use of NaOH in methanol/dichloromethane or The hydrolysis of amides and nitriles is a well studied reaction and numerous methods have. |
|
Untitled
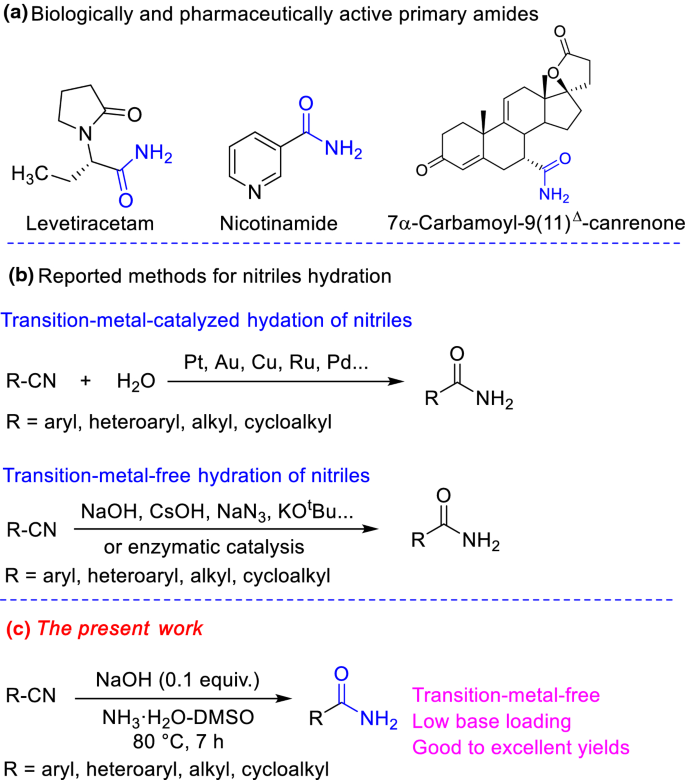
A facile conversion of nitrile to amide may be achieved by the reaction in the At high alkanity e.g. |
|
Reactions of Amines
H-X (proton acid). NaOH amine base ammonium salt. (acidic) Reaction with Ketones or Aldehydes (Section 18-1617 and 19-10). |
|
Kinetics and Equilibria of Amide Formation in Aqueous Media
methylamine reaction is on the average lower by 5 kcal./mole. containing 30 ml. of solutions 0.01 M in both NaOH and amide were placed into a. |
|
THE FORMATION OF AMMONIA FROM PROTEINS IN ALKALINE
of each reaction mixture were sealed in a series of small Pyrex tubes It is interesting to note that M NaOH hydroylzes the amide groups. |
|
Kinetic Aspects of the Alkaline Hydrolysis of Poly(acrylamide)
the residual amount of unreacted amide group. location of the first reaction of amide groups in ... simple second-order rate equation: 1 NaOH 0.25. |
|
EFFECTS OF CHEMICAL MODIFICATIONS ON POLYESTER FIBRES
sodium hydroxide solution on polyester fibres at various alkali concentrations and various Reaction of an amine with an ester group of the poly. |
|
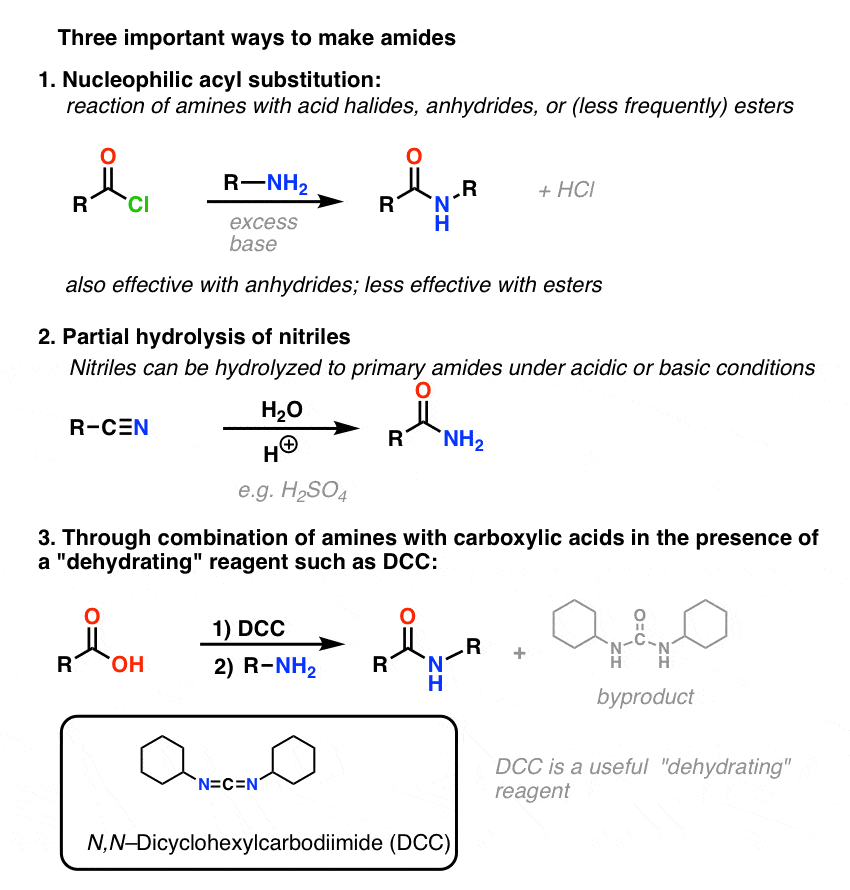
Chem 360 Jasperse Ch. 20 21 Notes + Answers. Carboxylic Acids
From Acid Chlorides Anhydrides |
|
Reaction of p-Toluenesulfonyl Isocyanate with Polymers Having
subjected to hydrolysis in a 1 M NaOH solution at 50 °C to convert 90% of the react with various nucleophiles such as amides. |
|
Experiment 13 – Properties of Amines and Amides
• Learn to recognize the amine and amide functional groups • Learn the IUPAC system for naming amines and amides • Learn the important physical properties of the amines and amides • Learn the major chemical reactions of amines and amides and learn how to predict the products of amide synthesis and hydrolysis reactions |
|
Chapter 19: Amines - University of Northern British Columbia
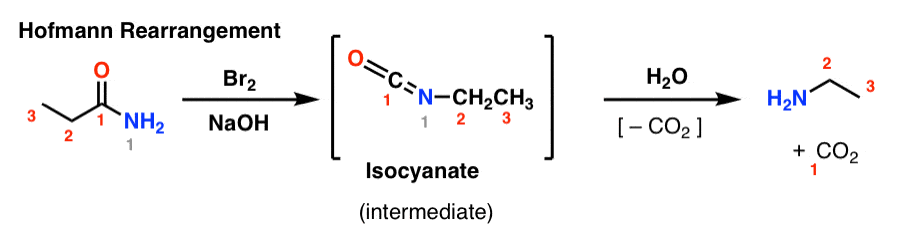
Primary amide react with bromine under basic condition to produce amines via a rearrangement reaction The reaction is limited to primary amide Secondary and tertiary do not give the reaction NH2 O NaOH Br 2/H 2O NH2 + CO2 NH O NaOH Br2/H2O no reaction 414 |
|
Reactions of Amines
Reactions of Amines Reaction as a proton base (Section 19-5 and 19-6) H-X(proton acid)HRN HamineNaOHbase RNHX Hammonium salt(acidic) Mechanism: Required (protonation) Reverse Mechanism: Required (deprotonation) Amines are completely converted to ammonium salts by acids Ammonium salts are completely neutralized back to amines by bases |
|
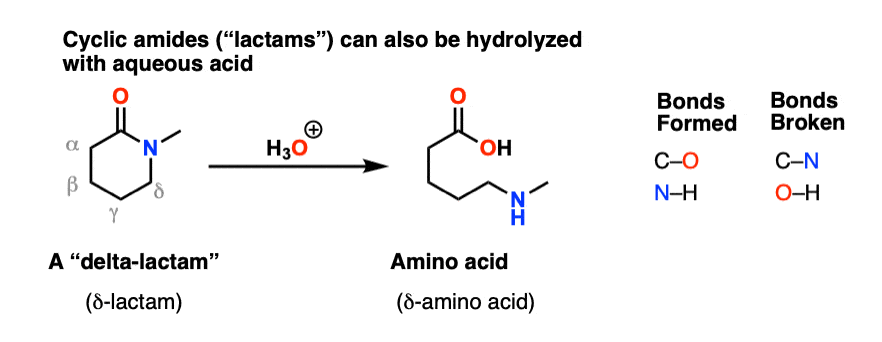
76 Hydrolysis of Amides - Oregon Institute of Technology
The reactive 4 amide ring is sometimes referred to as a ?-lactam ring Amides in rings are referred to as lactams This drug reacts with and destroys the transpeptidase enzymes in bacteria which crosslink the bacteria’s peptidoglycan cell wall ( a wall containing both peptide bonds and sugars) |
|
Searches related to reaction of amide with naoh filetype:pdf
A brief list of recommended reaction conditions for catalytic hydrogenations of selected functional groups is given below Catalyst/Compound SubstrateProductCatalystRatio (wt )Pressure (atm) AlkeneAlkane5 Pd/C5-10 1-3 AlkyneAlkene5 Pd(BaSO4)2 + 2 quinoline 1 Aldehyde(Ketone)AlcoholPtO2 2-4 1 |
What happens when an amine reacts with an acid?
- Amines characteristically react with acids to form ammonium salts; the nonbonded electron pair on nitrogen bonds the hydrogen ion: Amine Ammonium Salt If an amine is insoluble, reaction with an acid produces a water-soluble salt.
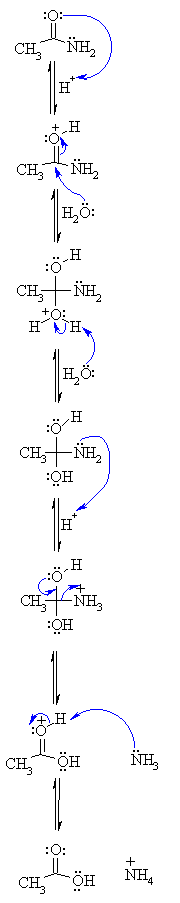
How do primary amides hydrolyze to carboxylic acid salts and ammonia?
- In base, primary amides hydrolyze to carboxylic acid salts and ammonia. The presence of ammonia (or amine from corresponding amides) can be detected similarly by odor or litmus. The carboxylic acid would be generated by neutralization with acid.
What is reactive 4 amide ring?
- 22 The reactive 4 amide ring is sometimes referred to as a ?-lactamring. Amides in rings are referred to as lactams. This drug reacts with and destroys the transpeptidase enzymes in bacteria which crosslink the bacteria’s peptidoglycancell wall ( a wall containing both peptide bonds and sugars).
What is an amide in a ring called?
- Amides in rings are referred to as lactams. This drug reacts with and destroys the transpeptidase enzymes in bacteria which crosslink the bacteria’s peptidoglycancell wall ( a wall containing both peptide bonds and sugars).
|
Reactions of Amines
This involves simple SN2, followed by deprotonation by the excess amine Br excess NH3 NH2 4 Acylation with Acid Chlorides to From Amides: (Section 19- 13 |
|
Chemistry N-DEACETYLATION OF SOME AROMATIC AMIDES
Then hydrolysis was carried out under classical vigorous reaction conditions and for a long reaction times using refluxing in 5N NaOH/H2O solution Aiming to |
|
THE HYDROLYSIS OF AMIDES IN THE ANIMAL BODY
evident that amide hydrolysis, being a relatively simple reaction, acid and of sodium hydroxide has now been the amide and HCI (or NaOH) were present |
|
The Amide Nitrogen Content of Gelatins - CORE
hydrolysis of amide side chains which thus give NaOH (6 g , A R ) were dissolved in1 1 of water, pre- products and free ammonia (browning reaction, see |

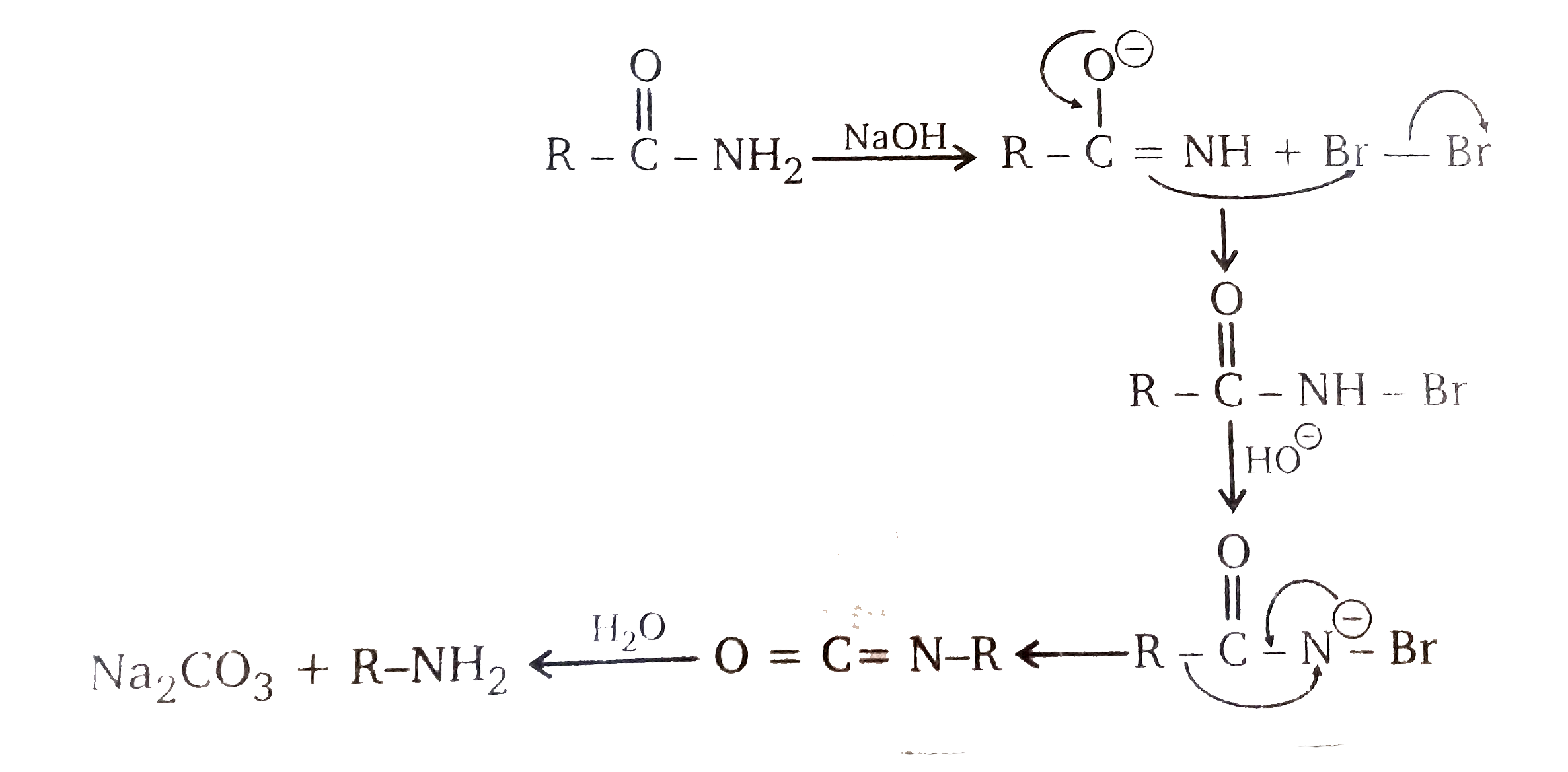








![SUMMARY - Reactions of Amines Phenylamine Amides - [PDF Document] SUMMARY - Reactions of Amines Phenylamine Amides - [PDF Document]](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/cf09decd-429c-4dd5-871c-a52710af5258/chem202001140-fig-0001-m.jpg)


