regulatory guidelines for software medical devices a lifecycle approach
|
Regulatory Guidelines for Software Medical Devices – A Lifecycle
To address this all software medical device manufacturers are recommended to adopt a Total Product. 52. Life Cycle (TPLC) approach to manage and adapt to |
|
Regulatory Guidelines for Software Medical Devices – A Life Cycle
2022?4?29? To address this all software medical device manufacturers are recommended to adopt a Total Product. Life Cycle (TPLC) approach to manage ... |
|
US FDA Artificial Intelligence and Machine Learning Discussion Paper
guidance) that describes a risk-based approach to assist in determining when a premarket submission is required.5. 1 Software as a Medical Device (SaMD): |
|
Guidance for Industry and FDA Staff Guidance for the Content of
2005?5?11? Submissions for Software Contained in Medical Devices issued May 29 |
|
General Principles of Software Validation; Final Guidance for
2002?1?11? Regulatory Requirements for Software Validation. ... consider the least burdensome approach in all areas of medical device regulation. |
|
Digital Health Regulation In Asia-Pacific
Telehealth Products” and “Regulatory Guidelines for Software Medical Devices – A Life Cycle. Approach.” Both of these guidance documents. |
|
Process Validation: General Principles and Practices - FDA
This guidance outlines the general principles and approaches that FDA considers 5 Guidance on process validation for medical devices is provided in a ... |
|
MDCG 2019-16 Rev.1
called the Medical Devices Regulations) have been adopted and entered into The overall approach for security management for medical devices and software ... |
|
Policy for Device Software Functions and Mobile Medical
To discuss an alternative approach contact the FDA staff or Office responsible for this guidance as listed on the title page. I. Introduction. The Food and |
|
Software as a Medical Device (SAMD): Clinical Evaluation Guidance
2017?6?22? FDA intends to consider the principles of this guidance in the development of regulatory approaches for SaMD and digital health technologies. In ... |
|
Regulatory Guidelines for Software Medical Devices – A Life Cycle
29 avr 2022 · To address this all software medical device manufacturers are recommended to adopt a Total Product Life Cycle (TPLC) approach to manage |
|
Regulatory Guidelines for Software Medical Devices – A Lifecycle
To address this all software medical device manufacturers are recommended to adopt a Total Product 52 Life Cycle (TPLC) approach to manage and adapt to |
|
HSA Guidance on Life Cycle Approach for Software Medical Devices
22 nov 2021 · The guidance provides non-binding recommendations to be considered by medical device manufacturers (software developers) and other parties |
|
HSA Guidance on Life Cycle Approach for Software Medical Devices
16 nov 2021 · The HSA has published a guidance document dedicated to the life cycle approach in the context of software medical devices |
|
Guidelines for Software as Medical Device and Artificial intelligence
5 THE REGULATORY REQUIREMENTS FOR MARKETING AUTHORIZATION OF SaMD medical devices and AI based medical devices in their entire life cycle |
|
A Hybrid Assessment Approach for Medical Device Software
This approach integrates agile methods into the medical device software development process whilst adhering to the requirements of the regulatory standards |
|
MEDICAL DEVICE REGULATIONS
Medical device regulations : global overview and guiding principles 1 Equipment and supplies – legislation 2 Equipment and supplies – standards 3 |
|
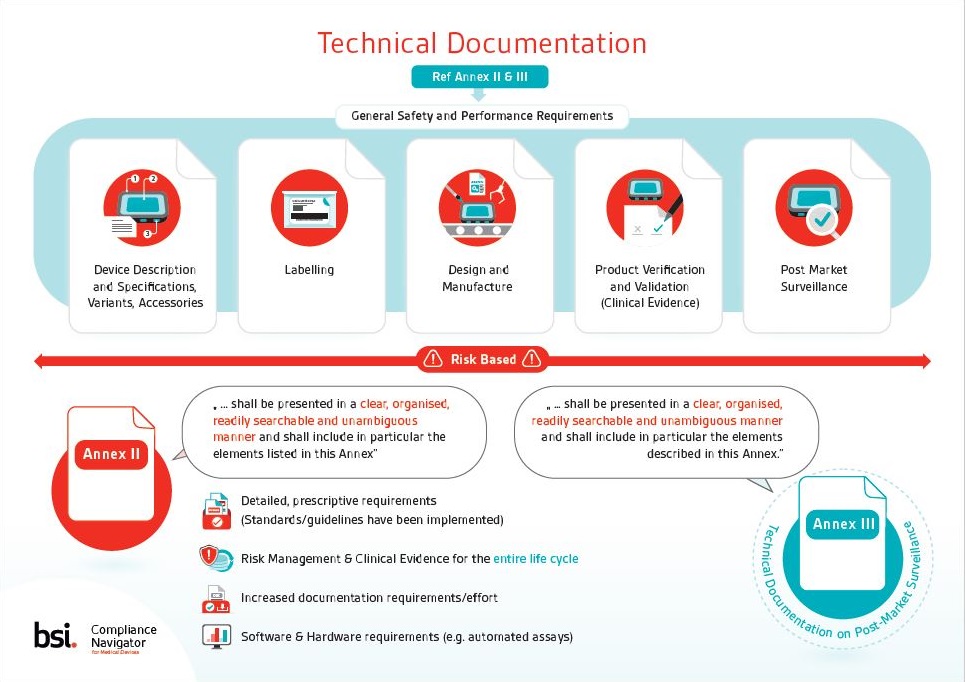
MDCG 2019-11 - Guidance on Qualification and Classification of
This document which primarily targets medical software manufacturers defines the criteria for the qualification of software falling within the scope of the |
|
MDCG 2019-16 Rev1 - Language selection Public Health
Table 2: Cybersecurity activities across the life cycle of medical devices according to the Medical Devices Regulations Pre-market activities |
What is a life cycle management of a medical devices?
The lifecycle management of medical equipment includes establishing policies and procedures for the use of the medical equipment, managing, and keeping track of equipment maintenance and repair jobs, and planning for replacements when the equipment reaches the end of its useful life.What is the regulatory approval process of medical devices?
Devices undergo laboratory and animal testing to answer basic questions about safety. Devices are tested on people to make sure they are safe and effective. FDA review teams thoroughly examine all of the submitted data related to the drug or device and make a decision to approve or not to approve it.What is the new medical device regulation?
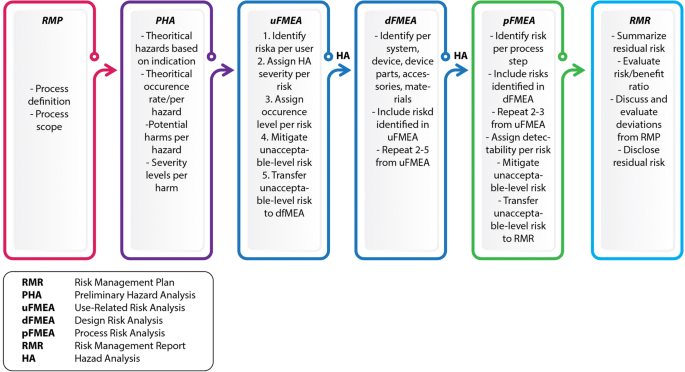
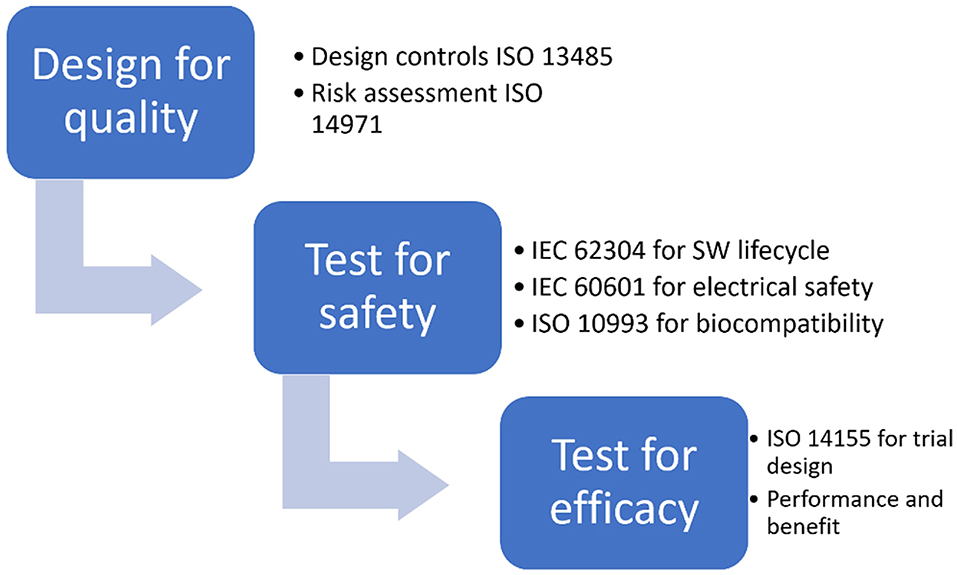
The new MDR regulation implies that it is no longer possible to market medical devices that contain or consist of viable biological material or viable organisms, including living micro-organisms, bacteria, fungi or viruses in order to achieve or support the intended purpose of the product.- ISO 14971 specifies the process for risk management of medical devices, software as a medical device (SaMD), and in vitro medical devices. The standard outlines a process for medical device manufacturers to identify hazards, evaluate the risks associated with them, and implement risk controls.
|
Regulatory Guidelines for Software Medical Devices – A Lifecycle
To address this, all software medical device manufacturers are recommended to adopt a Total Product 52 Life Cycle (TPLC) approach to manage and adapt to |
|
Regulatory Guidelines for Software Medical Devices – A Life Cycle
The international standard: ISO 13485 – Medical Devices – Quality Management Systems – Requirements for regulatory purposes, specifies requirements for a QMS that can be adopted by an organization involved in one or more stages of the life cycle of a medical device |
|
Software in Medical Devices - AdvaMed
Medical Device Software Non-Medical Device Software FDA Regulations and Guidance validation, including identification of the design, method(s), the date 62304 Medical Device Software- Software life cycle processes Standards |
|
AI/ML - FDA
Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device The 510(k) software modifications guidance focuses on the risk to these tools requires a new, total product lifecycle (TPLC) regulatory approach that facilitates a |
|
Software as a Medical Device (SaMD) - APACMed
25 jui 2020 · Considerations in Development, Validation, and Lifecycle Management Quality of Data Inputs Alternative Approach for Modifications to AI Software 18 HSA Regulatory Guidelines for Software Medical Devices Source: |
|
MEDICAL DEVICE REGULATIONS - WHO World Health Organization
Medical device regulations : global overview and guiding principles 1 Equipment and The health technology life cycle diagram (back cover) implant, in vitro reagent or calibrator, software, material or other similar or related article, intended by the but for which there is not yet a harmonized approach, are: • aids for |
|
Algorithms as medical devices - PHG Foundation
Appendix 3: Harmonised standards relevant to medical device software 42 in force in 2020 and 2022) emphasises a life-cycle approach to regulating medical |
|
MDCG 2019-11 - European Commission - Europa EU
Annex II - Qualification examples of Medical Device Software (MDSW) 1 The use of “The Medical Devices Regulations” from here on out refers to both MDSW that provides insulin dose recommendations to a patient regardless of the method of manufacturer shall ensure safety and performance throughout the lifecycle |
|
Software Development for Medical Devices - Boundary Systems
used in medical devices fall under regulatory scrutiny Two prominent Regulations (as well as ISO 13485 specifications) defines a With any product lifecycle, change is inevitable In want to leverage the software product lines approach |