secondary amine reaction with water
|
Kinetics reaction of primary and secondary amine group in aqueous
Both DETA and water contribute to the carbamate formation in the primary and secondary amine groups. The rate of CO2 absorbed in the primary amine group is |
|
Nucleophilicities of Primary and Secondary Amines in Water
Plots of log k2N for these reactions vs the electrophilicity parameters E of the benzhydrylium ions were linear |
|
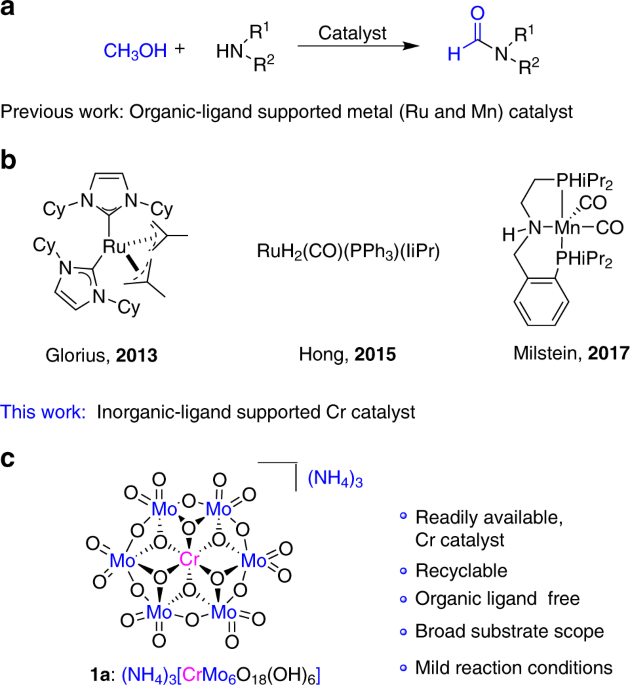
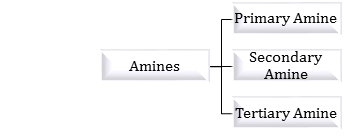
Chapter 6 Amines and Amides
secondary (2°) amine. (-amine). NH2 tertiary (3°) amine. (-amine) quaternary ammonium Reaction of an amine with water to produce an alkylammonium ion. amine. |
|
REACTION OF TRICHLOROACETONITRILE WITH PRIMARY AND
VV. lltansrr (Herrliberg Switzerland). that the salts of the primary and secondary amines do not react with trichloroacetonitrile in water solution |
|
A perspective approach on the amine reactivity and the hydrogen
the three amine states observed during epoxy-amine reaction (primary amine β-hydroxylamine as secondary amine |
|
NOTE A Novel Four-Component Reaction Between a Secondary
amine in the presence of water proceeds smoothly at room temperature to reaction between isocyanide 4 secondary amine 2 and an electron-poor ... |
|
On the reaction of glycidol with a secondary amine
Using secondary aliphatic amines it is possible to pre- pare valuable water-soluble polymers resulting from the following set of reactions where R denotes |
|
Assessing the Potential for the Reactions of Epoxides with Amines
Sep 18 2558 BE the amine−epoxide reactions were found to be water-catalyzed and not ... Secondary Organic Aerosol in the Reactions of Aliphatic Amines with. |
|
Sampling of diisocyanates (HDI TDI) in air by derivatisation with
However water or alcohols react orders of magnitude more slowly with isocyanates isocyanates with reagents of the secondary amine type show significant ... |
|
Reactions of Polyfluoro Olefins. II.1 Reactions with Primary and
alcohols amines and water. Analytical data gave an empirical formula of C«Hi<iC1F2N reaction of secondary amines with chlorotri- fluoroethylene or with ... |
|
Aminolysis of acid anhydrides in water. II. Nonlinear structure
Abstract: The reactions in water of 21 primary and secondary amines with phthalic and succinic anhydrides were examined. Each reaction was first order in |
|
REACTION OF TRICHLOROACETONITRILE WITH PRIMARY AND
Trichloroacetonitrile reacts with primary and secondary aliphatic and primary amines were allowed to react with trichloroacetonitrile in water-methanol ... |
|
Kinetics reaction of primary and secondary amine group in aqueous
The reaction of the secondary amine group of DETA with CO2 is shown in reaction (109):. (10). If in the carbamate formation reactions |
|
Chapter 6 Amines and Amides
Learn the major chemical reactions of amines and amides and learn how to predict the Amines are classified as primary (1°) |
|
Formylation of Amines
10 juin 2014 amines diamines |
|
Nucleophilicities of Primary and Secondary Amines in Water
Plots of log k2N for these reactions vs the electrophilicity parameters E of the benzhydrylium ions were linear |
|
Aminated poly(ethylene glycol) methacrylate resins as stable
exhibit any turnover indicating that the secondary amine in the backbone of Keywords: Aminated PEGMA |
|
Secondary amine selective Petasis (SASP) bioconjugation
of the Petasis reaction for secondary amines in contrast to primary amines and ammonia; Next peptide PAF 1a and SAL 2a were exposed to more water. |
|
Secondary amine selective Petasis (SASP) bioconjugation
S1†). Next peptide PAF 1a and SAL 2a were exposed to more water soluble p-methoxyphenyl boronic acid PMB 3b and reaction was. |
|
Assessing the Potential for the Reactions of Epoxides with Amines
18 sept. 2015 the amine?epoxide reactions were found to be water-catalyzed and not ... amine secondary organic aerosol (SOA) concentrations ... |
|
Introduction to Amines - CliffsNotes
Physical Properties of Amines: Water Solubility • 1° 2° and 3° amines can all form hydrogen bonds with water • Low-molecular weight amines are generally water-soluble CH3 N H H H O H O H H O HH CH3 N CH3 H H O H O H H CH3 N CH3 CH3 H O H 20 Physical Properties of Amines: Odor • Low molecular-weight amines tend to have sharp |
|
Amines - Rutgers University
The free amines are generally insoluble in water but soluble in organic solvents This provides an excellent method for the separation and isolation of amine compounds Free amines are insoluble in water but when dilute acid is added the ammonium salt is produced which dissolves |
| Covalent Scavengers for Primary and Secondary Amines - Thieme |
What happens to amines in water solution?
- Amines in water solution exist as ammonium ions. In water, the ammonium salts of primary and secondary amines undergo solvation effects (due to hydrogen bonding) to a much greater degree than ammonium salts of tertiary amines.
Which reaction gives primary amine and secondary amine?
- On reduction one gives primary amine and the other gives secondary amine. Identify the class of compounds. Explain the reduction reaction. Cyanides and Isocyanides.
What happens when amine reacts with NaOH?
- Amines, being basic in nature, react with acids to form salts. Amine salts on treatment with a base like NaOH, regenerate the parent amine. Amine salts are soluble in water but insoluble in organic solvents like ether.
What is the oxidant of secondary amines in organic solvents?
- The most effective and frequently used oxidant of secondary amines in organic solvents (CH2CI2, CH3CN, MeOH) is m- chloroperbenzoic acid (m-CPBA). Oxy-dation with m-CPBA of seco-curane type indoline alkaloids of strychnobrasiline (32), in deacetylated form, gives the corresponding nitrones (33), (34), and (35) (Scheme 2.12) (86). [Pg.138]
|
Reactions of Amines
Reverse Mechanism: Required (deprotonation) • Amines are completely converted to ammonium salts by acids • Ammonium salts are completely neutralized |
|
Kinetics reaction of primary and secondary amine group in aqueous
Both the termolecular and the zwitterion mechanisms were applied to interpret the experimental data and gave identical result Both DETA and water contribute to |
|
CHAPTER 7 AMINES
Naming Secondary and Tertiary Amines When there is more Amines undergo neutralization reactions with acids to form alkylammonium ions a) CH3NH2 |
|
NITROGEN CONTAINING COMPOUNDS Amines - Patna Science
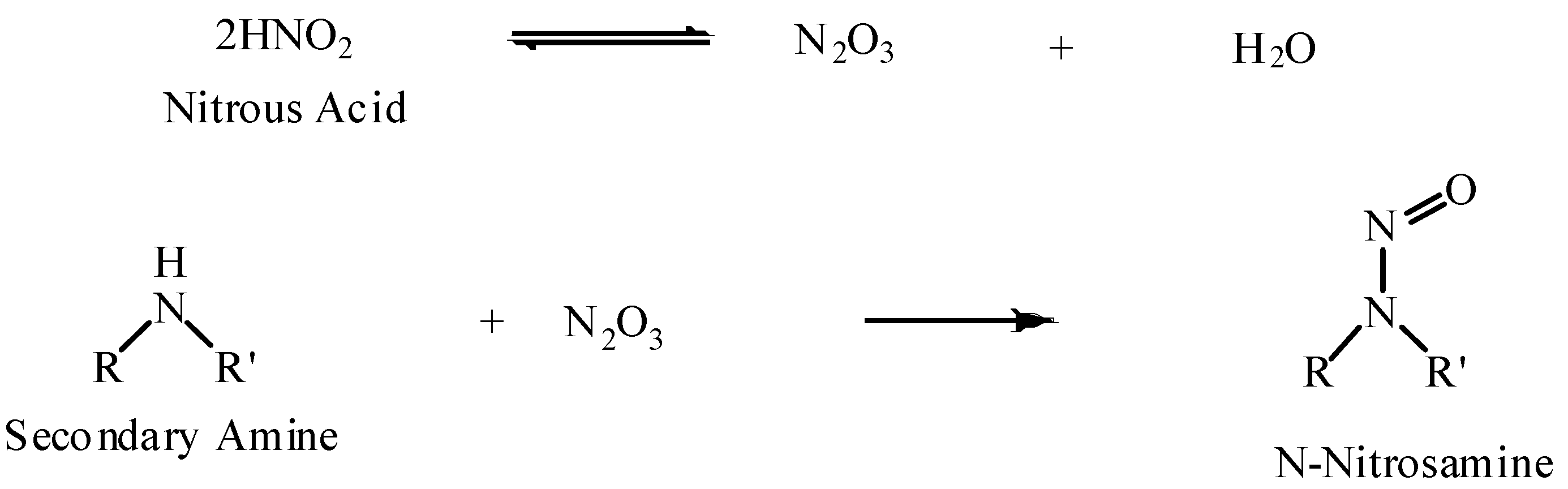
mixture of an amine and a water-insoluble but ether- soluble ketone can be (b) Secondary amines react with nitrous acid to form N-nitrosamines which are |
|
Amines
What Are the Reactions of Amines with Acids? 10 6 How Are alkyl or aryl groups Amines are classified as primary (1°), secondary (2°), or tertiary (3°), |
|
AMINES
and Ammonia All secondary amines react with carbon disulfide in ammoniacal solution with the formation of ammonium salts of dithiocarbamic acids, which do |
|
Amines Amines - NCERT
secondary and tertiary amines, when two or more groups are the same, the prefix di or tri is Amines, being basic in nature, react with acids to form salts |
|
AMINES
29 mar 2020 · Amines are classified as primary, secondary or tertiary depending In reaction ( 1) any structural feature that stabilizes the ammonium ion (relative to the it decreases its ability to form hydrogen bond with water molecules |
|
Amine Chemistry Tutorial
Amines are sub-classified as primary, secondary and tertiary based on the degree of Because of their basicity, amines react with acids to form salts |
















![PDF] Synthesis of primary amines from secondary and tertiary PDF] Synthesis of primary amines from secondary and tertiary](https://cdn.britannica.com/s:300x169)