standard free energy change in biochemical reaction pdf
|
Bioenergetics Free Energy Change
Standard free energy change ?Go is when reaction conditions are standard: T is 25 Biochemical reactions are usually coupled to the hydrolysis of ATP:. |
|
Untitled
13.3 Biological Oxidation-Reduction Reactions 507 heat and that this process of rium is defined as the standard free-energy change AG. |
|
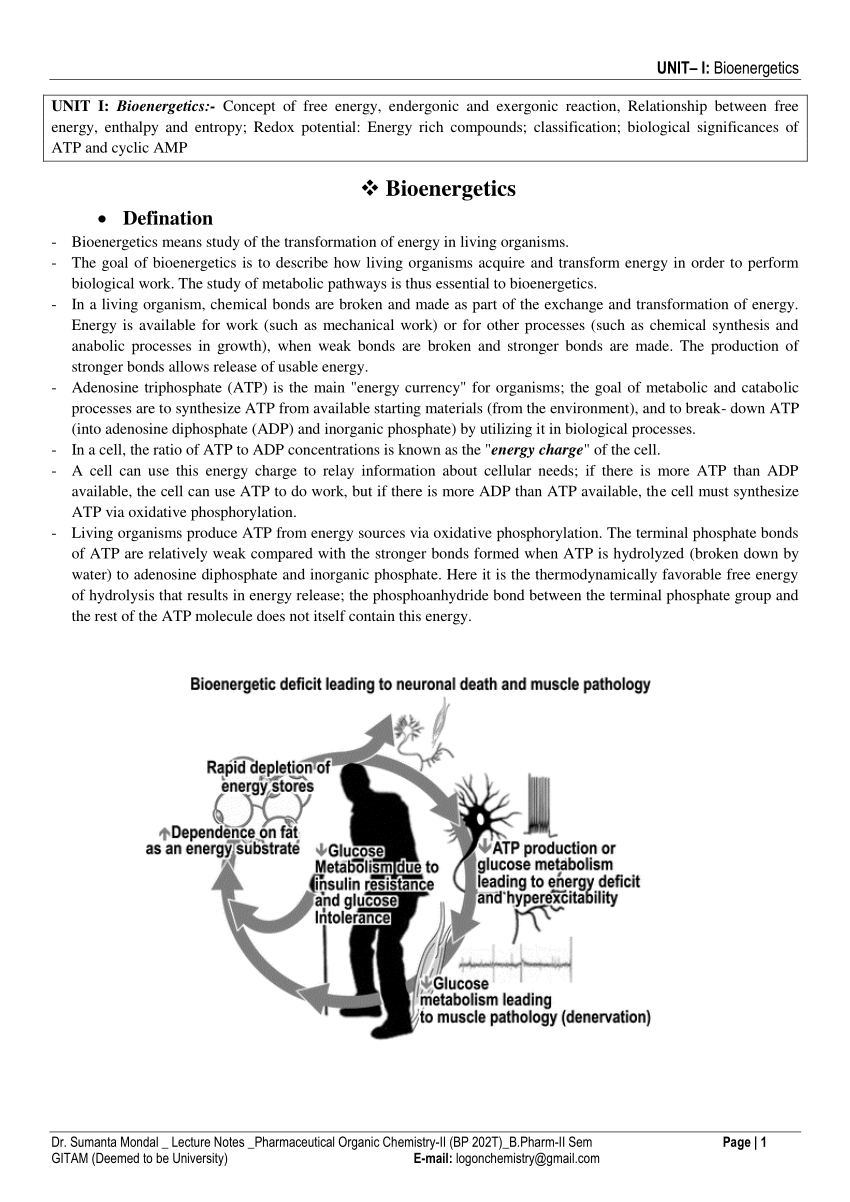
Bioenergetics
Standard Free Energy. Changes at pH 7.0 or. ?G °?. • ATP as Universal Currency of. Free Energy in Biological. Systems. • Free Energy of Hydrolysis of. |
|
The estimation of the apparent standard free energy change Î?G
Energy Change AG°pH of a Biochemical Reaction from the Standard Free Energy of Formation and. Apparent Free Energy of Ionization of the Participat-. |
|
Quantum Mechanical Methods Predict Accurate Thermodynamics of
25 mar. 2021 standard Gibbs free energy change of biochemical reactions ... thermochemical parameters for diverse biological reactions. |
|
The Free Energy Change in Hydrolytic Reactions: The Non-ionized
all freeenergy change associated with the hydrolysis of a number of compounds at a fixed pH value can “Energetics in Biochemical Reactions” Academic. |
|
DGPredictor: Automated fragmentation method for metabolic
29 mar. 2021 pathways to estimate the standard Gibbs free energy change ... for the prediction of standard Gibbs free energy of biochemical reactions. |
|
The use of standard free energy of formation values in the
of energy from catabolic to anabolic reactions. Most biochemistry texts use the ... Pi) the magnitude of the standard free energy change for the. |
|
PowerPoint Template
Free Energy Change for Biological. Reactions. ? Thermodynamic quantities describing the energy changes in chemical reaction. ? Gibbs free energy G. |
|
Bioenergetics Free Energy Change - CSUN
The free energy change (?G) of a chemical process is a measure of the energy available to do work • Standard free energy change ?Go is when reaction |
|
Principles of - biochemistry
Because the standard free-energy change is negative when the reaction starts with 1 0 M glucose 1-phosphate and 1 0 M glucose 6-phosphate the conversion of |
|
Semester 2 (CC3) Unit 1- Bioenergetics - Hooghly Womens College
We can obtain the standard free energy change for the overall reaction by adding the standard free energy changes for all the individual reactions This |
|
Bioenergetics
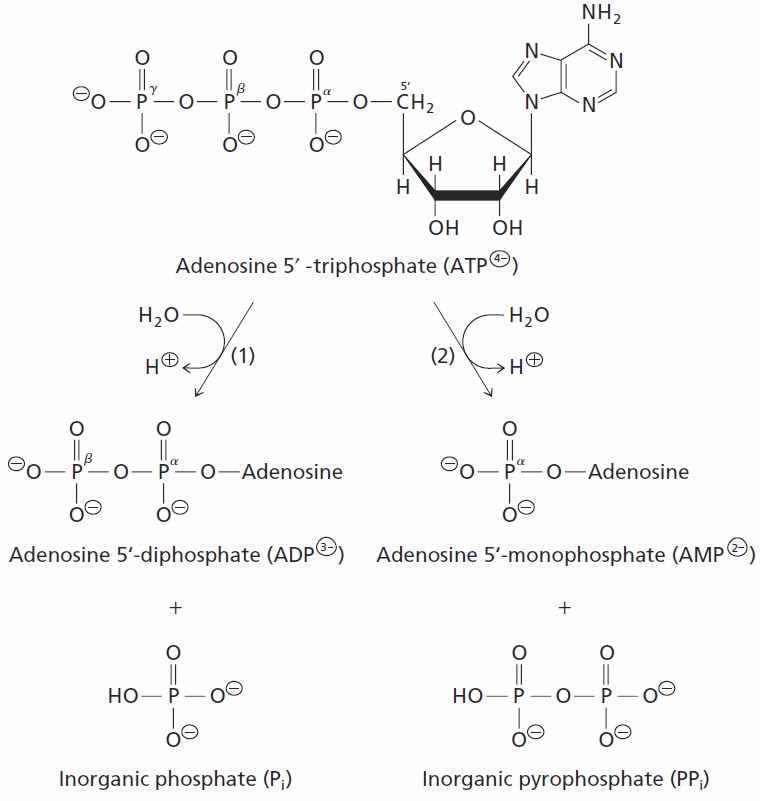
ATP + H2O ? ADP + Pi The standard free energy change ?G°? for this reaction is –7 3 kcal/mol Standard free energy changes have also been determined for |
|
Biochemical Thermodynamics - Jones & Bartlett Learning
State and appropriately use equations relating the free energy change of reactions the standard-state free energy change the equilibrium constant and the |
|
CHAPTER 13
CHAPTER 13 Bioenergetics and Biochemical Reactions Types 1 Standard free energy change is directly related to the equilibrium constant |
|
262: Free Energy Changes in Chemical Reactions
24 déc 2022 · In a spontaneous change Gibbs energy always decreases and never increases This of course reflects the fact that the entropy of the world |
|
Chapter 13 – Bioenergies and Biochemical Reaction Types
Page 1 Chapter 13 – Bioenergies and Biochemical Reaction Types Standard Free-Energy Change is Directly Related to the Equilibrium Constant |
|
Concept of Free Energy - Patna Womens College
When a reaction proceeds with the release of free energy (that is when the system changes so as to possess less free energy) the free-energy change G has a |
|
Free energy changes in biochemical systems
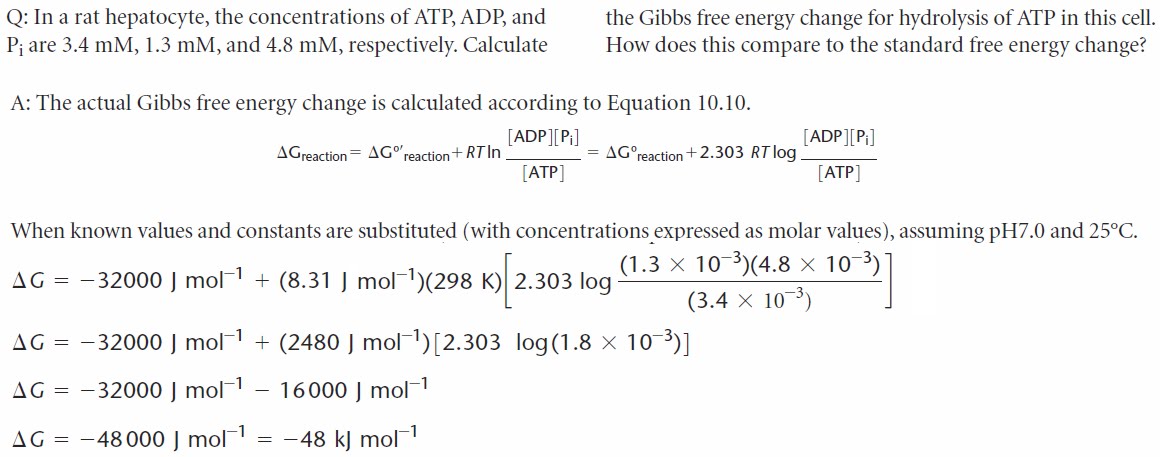
The difference between standard free energy change and the actual free energy free energy change for a chemical reaction was described by the equation: |
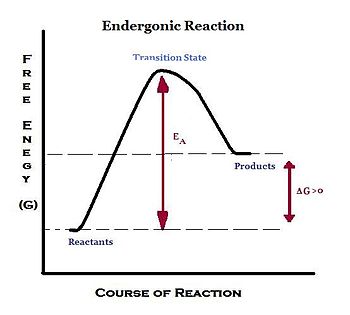
What is the standard free energy change in a biochemical reaction?
For biochemical reactions, the standard free-energy change is usually expressed as ?G°?, which is the standard free-energy change of a reaction in aqueous solution at pH= 7, approximately the conditions within a cell.What is the standard biochemical free energy change of ATP hydrolysis?
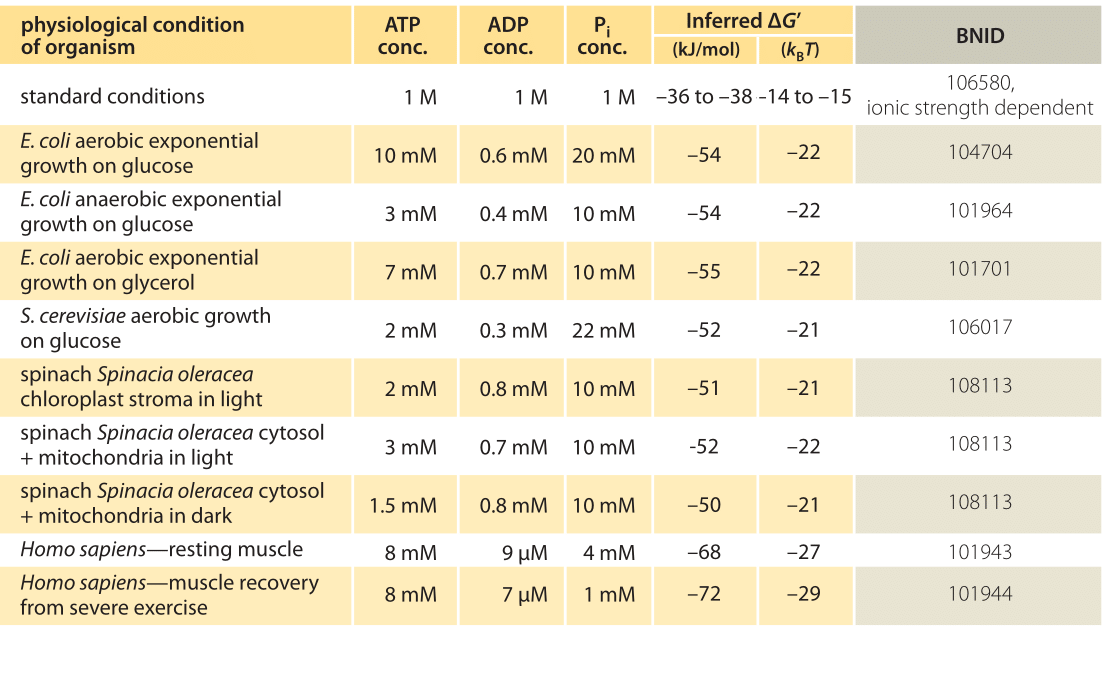
Under “standard” conditions (i.e. concentrations of 1M for all reactants except water which is taken at its characteristic concentration of 55M) the Gibbs free energy of ATP hydrolysis varies from -28 to -34 kJ/mol (i.e. ?12 kBT, BNID 101989) depending on the concentration of the cation Mg2+.What is the concept of free energy and standard free energy change in bioenergetics?
Free energy in bioenergetics is the amount of energy that is capable of doing work; the amount of available energy. It has a simple calculation. The change in free energy = change in enthalpy - (temperature times change in entropy). Temperature unit is Kelvin in this equation.- The ?G°' term is called the change in Standard Gibbs Free energy, which is the change in energy that occurs when all of the products and reactants are at standard conditions and the pH is 7.0. It is a constant for a given reaction. For most biological systems, the temperature, T, is a constant for a given reaction.
|
Bioenergetics Free Energy Change - CSUN
Standard free energy change ∆Go is when reaction conditions are Biochemical reactions are usually coupled to the hydrolysis of ATP: ATP ←→ ADP + Pi |
|
Biochemical Thermodynamics - Jones & Bartlett Learning
dissociation constant, standard state, and biochemical standard state 2 State and appropriately use equations relating the free energy change of reactions, the |
|
II Bioenergetics - University of Lethbridge
Biological energy transformations obey the laws of thermodynamics 1 You can't Each chemical reaction has a characteristic standard free-energy change |
|
BIOENERGETICS Bioenergetics or biochemical - Yengage
Bioenergetics or biochemical thermodynamics deals with the study of energy changes (transfer and the mechanism of chemical reactions concept was first developed by Willard Gibbs in 1870s and hence free energy is denoted as ΔG The free energy change becomes zero (AG = 0) when a reaction is at equilibrium |
|
Gibbs Free Energy
27 jan 2010 · Molecular Biology and Biochemistry of the Cell Lecture 6 How Gibbs Free Energy can be used to determine How a reaction occurs (mechanism) 3 Rate at which a For all changes in a system, the total entropy of the |
|
Chem331 Lect 17 Thermodynamics - University of San Diego Home
Second law of thermodynamics – Entropy must increase if a reaction is to be To compare thermo parameters of different reactions we define a standard state most biological/biochemical processes entropy changes are more useful than |
|
Lehninger-Ch13_smallpdf - chemuwecedu
Biological energy transductions obey the same physical laws that govern all other natural reaction proceeds with the release of free energy (that is, when the system The Standard Free-Energy Change Is Directly Related to the Equilibrium |
|
Levels of thermodynamic treatment of biochemical reaction - CORE
implicit in the mechanism of chemical change (Smith and Missen, 1982) standard Gibbs energy of reaction is related to the equilib- rium constant K by |
|
Bioenergetics
Bioenergetics, or biochemical thermodynamics, is the study Gibbs change in free energy (∆G) is that For biochemical reactions, a standard state is defined |
|
Standard Gibbs Free Energy, Enthalpy, and Entropy Changes as a
concentrations means that the chemical potentials p of the ions are given by the standard enthalpy changes, AHiO, of these ionic reactions by use of (20) |










90187-6.fp.png)



