supersaturated solution definition
|
Cluster size estimation in binary supersaturated solutions
Substituting in the definition of M~we have fraction p |
|
Sodium chloride precipitation reaction coefficient from crystallization
Aug 29 2017 At initial time |
|
Bubble growth behavior in supersaturated liquid solutions
Supersaturation ratios less than 100 are most commonly encountered. The supersaturation ratio is defined as the ratio of the actual amount of dissolved gas in |
|
Test Definition: SUPRA
Supersaturation Reference Means (Delta G: DG). Brushite: 0.21 DG of transfer from a supersaturated to a saturated solution.(Werness PG Brown ... |
|
The Effect of a Visually-Based Intervention on Students’
supersaturated solution as the saturated solution and vice versa. Of the 17% who chose the. Page 5. correct order of definitions for the solutions only 4 |
|
9.5 PRECIPITATION The terms defined in this sub-chapter are
saturated solution at the same temperature and pressure. Supersaturation (noun). The state of supersaturated solution. Nucleus. The smallest solid phase |
|
A definition of lithogenic bile
supersaturated solution await further delineation. They perhaps ultimately hold the key to a better understanding of the process of the human gallstone. |
|
What Is the Critical pH and Why Does a Tooth Dissolve in Acid?
If the pH of the solution is above the critical pH then the solution is supersaturated with respect to the means that the tooth will begin to dissolve. |
|
Untitled
Definition of Solubility: the maximum amount of a substance that will dissolve in a Supersaturated Solution (a solution that has been heated so a very high. |
|
AP* Chemistry PROPERTIES OF SOLUTIONS
➢ Supersaturated solution—oxymoron—a solution that has been prepared at an A solution does not have a sharply defined freezing point a solvent does. |
|
Supersaturated Solution Science Definition
Recognizing the pretension ways to get this book Supersaturated Solution Science Definition is additionally useful. You have remained in right site to start |
|
Definition Of Supersaturated Solution
Definition Of Supersaturated Solution is easy to use in our digital library an online access to it is set as public fittingly you can download it instantly. Our |
|
Supersaturated Solution Science Definition
Right here we have countless book Supersaturated Solution Science Definition and collections to check out. We additionally offer variant types and as a |
|
Read Free Definition Of Supersaturated Solution ? - covid19.gov.gd
27-Aug-2022 Definition Of Supersaturated Solution is available in our book collection an online access to it is set as public so you can. |
|
Download File PDF Definition Of Supersaturated Solution Copy
We come up with the money for. Definition Of Supersaturated Solution and numerous ebook collections from fictions to scientific research in any way. in the |
|
Read Free Definition Of Supersaturated Solution - covid19
Definition Of Supersaturated Solution. Eventually you will very discover a new experience and talent by spending more cash. still when? do you understand |
|
Access Free Supersaturated Solution Science Definition ? - covid19
in the midst of guides you could enjoy now is Supersaturated Solution Science Definition below. A Dictionary of Science Literature |
|
The Influence of Polymers on the Supersaturation Potential of Poor
21-Sept-2018 The degree of supersaturation is defined as the ratio between the activity of the dissolved drug in a supersaturated solution and the ... |
|
Supersaturation-Based Drug Delivery Systems: Strategy for
06-May-2022 medicines resulting in supersaturated drug solutions |
|
Supersaturated Solution
2016 Flinn Scientific. Supersaturated Solution. Sodium Acetate Demonstration. Continued on page 2. Introduction. Snap your fingers over a clear solution. |
|
Supersaturated Solution - Definition Examples & Applications with
Supersaturated Solution - A supersaturated solution is one in which more solute is dissolved than is necessary to make a saturated solution |
|
Solutions
A solution that is more concentrated than that of a saturated solution is called as supersaturated solution at that particular temperature Solution may be |
|
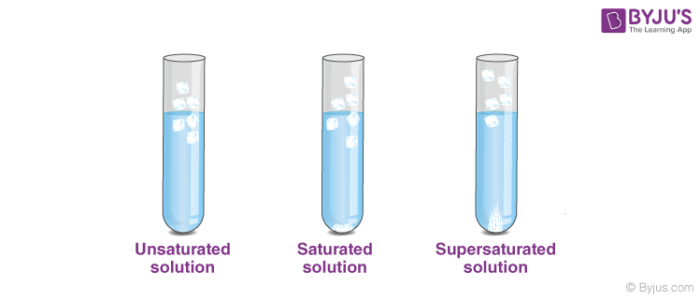
Saturated Unsaturated and Supersaturated Solutions
Supersaturated Solution: a solution that contains more solute than the solvent is capable of dissolving The undissolved solute tends to crystallize and |
|
Supersaturated Solution - Crystallization Condensation - Vedantu
A Supersaturated Solution is defined as the excess solute solution that can be dissolved by the solvent If we are not aware of what a solute or solvent is the |
|
Supersaturated Solution Definition and Examples - Science Notes
3 fév 2022 · Usually supersaturation involves a solid solute dissolved in a liquid solvent but the term also applies to gases in liquids and gas mixtures |
|
Supersaturated Solutions
In this experiment you will look at a very special case of solubility the creation of a supersaturated solution of sodium acetate trihydrate Na2C2H3O2 3H2O |
|
Supersaturated Sodium Acetate Solution - Flinn Scientific
A supersaturated solution can be made by gradually cooling a saturated solution without agitation so that crystals do not form Supersaturated solutions are |
|
Supersaturated Solution: Learn its Example Preparation Uses
27 sept 2022 · An example of a supersaturated solution is carbon in soft drinks Due to pressure more than the maximum carbon is dissolved into the solvent |
What is the definition of supersaturated in chemistry?
Supersaturation is defined as the difference between the solute concentration (c) and its equilibrium concentration under the same conditions (c*), that is, its solubility in solution.What is the definition of supersaturated and examples?
Answer: A solution that contains more amount of solute than that of saturated which is able to dissolve in solvent at a particular given temperature. This is the meaning of supersaturated solution. A significant example for supersaturated solution is sodium acetate.Why is a solution supersaturated?
How Does a Solution Become Supersaturated? A solution becomes supersaturated when the amount of solute per volume of solution exceeds its maximum theoretical concentration. That is when the solute concentration exceeds its solubility.- Carbonated Drinks.Lemonade with too much sugar.Honey.A mixture of powdered soap and water with too much soap.Butter oversaturated with salt.Water oversaturated with cocoa powder.Coffee oversaturated with creamer powder.Maple Syrup.
|
Solutions
13 Definitions SUPERSATURATED SOLUTIONS • contain more solute than is possible to be dissolved Supersaturated solutions are unstable and temporary |
|
What Is A Supersaturated Solution In Science
Supersaturated Solution - Definition, Examples A supersaturated solution is a solution with more dissolved solute than the solvent would normally dissolve in |








![Unsaturated saturated and supersaturated solutions - [PDF Document] Unsaturated saturated and supersaturated solutions - [PDF Document]](https://image.slidesharecdn.com/unsaturatedsaturatedandsupersaturatedsolutions-141002072749-phpapp02/95/unsaturated-saturated-and-supersaturated-solutions-10-638.jpg?cb\u003d1412234998)











