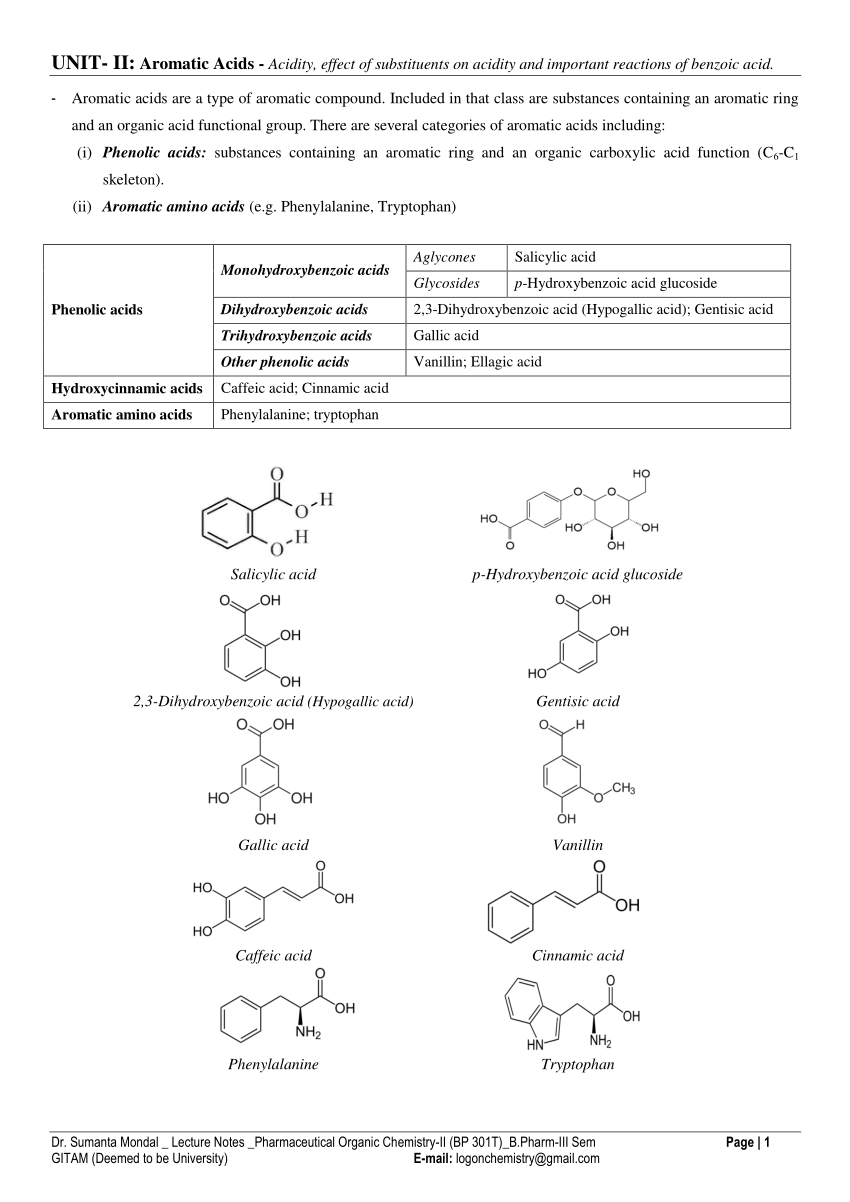
test for carboxylic acid and phenol
|
Identifying an Unknown Compound by Solubility Functional Group
In this lab we will focus on using Solubility Tests Chemical Tests and The reactions of carboxylic acids and phenols are shown in Equations 1 and 2 |
|
Lab 14: Qualitative Organic Analysis
Both strong and weak acids (Carboxylic acids and phenols) will be Phenols. Bromine water. C-8. Tests for the presence of phenols & aromatic amines. |
|
Testsforfunctionalgroups - inorganiccompounds
glacial acetic acid. TESTS FOR FUNCTIONAL GROUPS IN ORGANIC COMPOUNDS ... This is a test that distinguishes carboxylic acids from phenols. |
|
Chapter 5 Carboxylic Acids and Esters
Learn the major chemical reaction of carboxylic acids and esters and learn how to carboxylic acid with an alcohol or phenol (plus an. |
|
Identification of Organic Compound by Organic Qualitative Analysis
(A) Perform the following test only if the substance is soluble /miscible in Group IV : C H |
|
Functional group detection
Test for carboxylic acid group Test for phenolic functional group: ... red/green/pink/blue-violet colours confirms the presence of phenolic functional ... |
|
Experiment 8 – Synthesis of Aspirin
The phenol group on the salicylic acid forms an ester with the carboxyl group on the acetic acid. However this reaction is slow and has a relatively low yield. |
|
PRACTICAL LAB MANUAL
Functional group test: test for carboxylic acid. 6. 4. Functional group test: test for Phenols are aromatic alcohol where the R group is aromatic ring. |
|
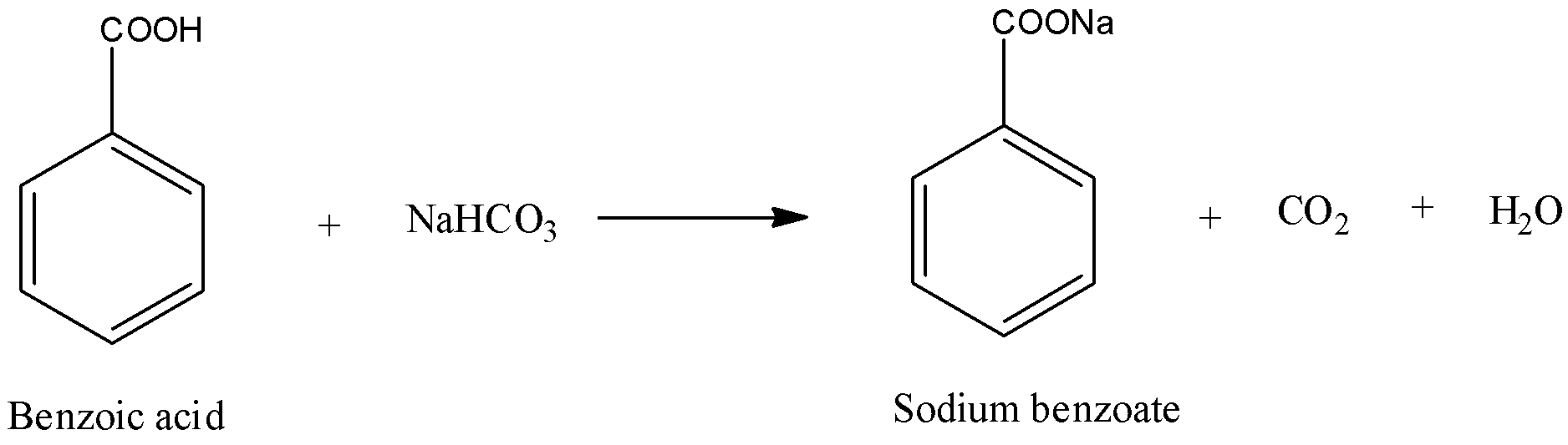
Acid-Base Extraction.
In the experiment done in this lab a mixture of a carboxylic acid (stronger acid) |
|
Identifying an Unknown Compound by Solubility Functional
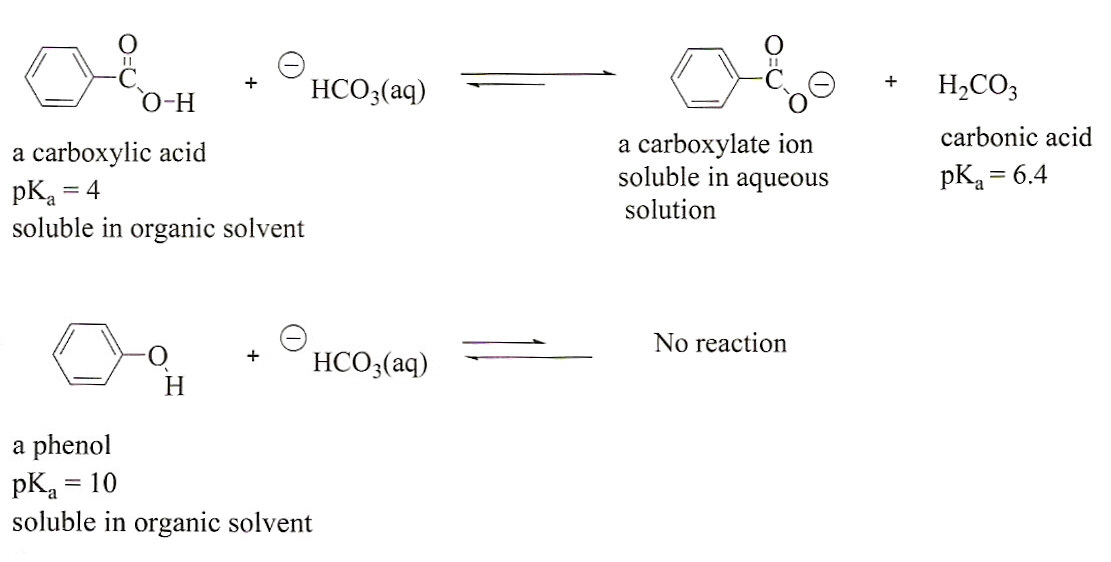
A pH of 4 or lower indicates a carboxylic acid A pH of 8 or higher indicates an amine Water-soluble compounds are tested with 5 sodium hydrogen carbonate (NaHCO3) to determine whether or not they are carboxylic acids Carboxylic acids react with NaHCO3 to produce carbon dioxide bubbles as shown below in Equation 3 |
|
Searches related to test for carboxylic acid and phenol PDF
1 Test for carboxylic acid group (a) Litmus test- Add blue litmus solution (1 drop) to an aqueous solution of acid appearance of a red colour indicates the presence of a carboxylic acid (blue litmus paper may be used in place of a blue litmus solution) (b) Sodium bicarbonate test (Functional group test): To a saturated solution of |
Table of Content
Aim Theory Apparatus Procedure Observations Results Viva-Voce Carboxylic acids are versatile organic compounds. It has excellent physical and chemical properties. The chemical structure of carboxylic acidcontains a carbonyl functional group and hydroxyl group. It interact easily with polar compounds and contributes to many important chemical reacti...
Theory
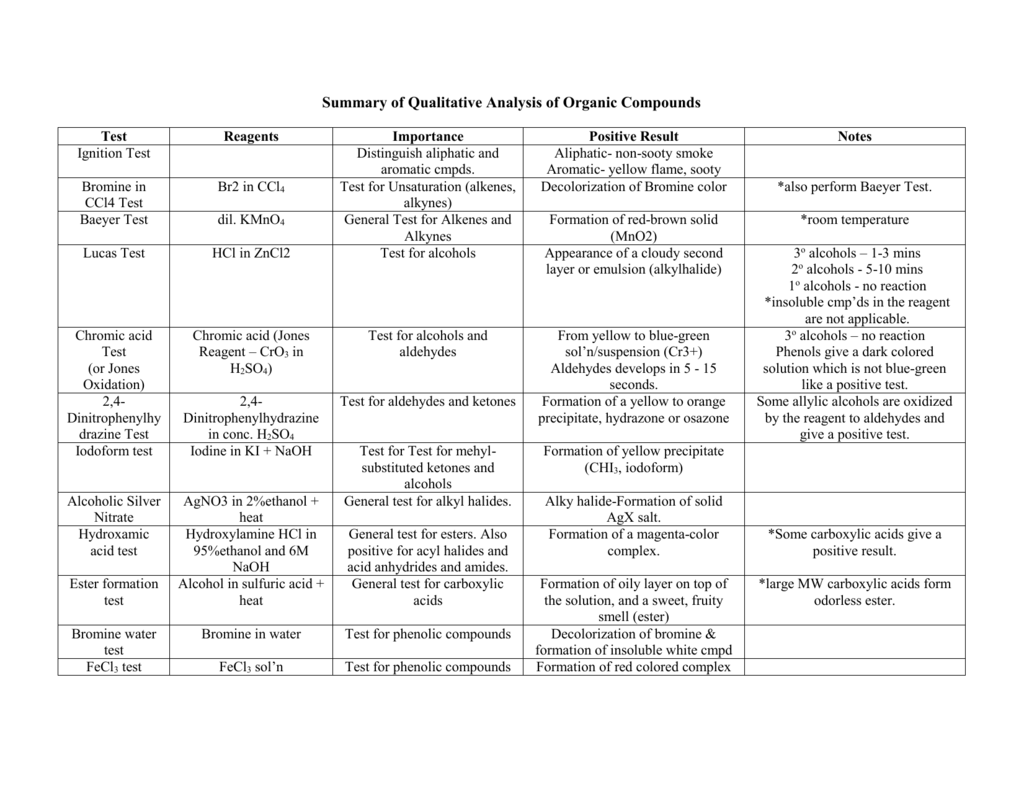
Carboxylic acids have a tendency to donate protons and act as acids. It is this property which is helpful in the identification of a -COOH group. Any of the following tests can be carried out to detect the carboxylic functional group. 1. Litmus test 2. Sodium bicarbonate test (or) Sodium hydrogen carbonate test 3. Ester test 4. Fluorescein test
Materials Required
Blue litmus paper
Procedure
(a) Litmus Test:
How do scientists test if phenol is acidic or basic?
Scientists use litmus paper to test whether the given solution is acidic or basic. Red litmus paper turns blue while blue litmus paper remains unchanged in the presence of a base. Phenol turns blue litmus paper red. This shows that phenol is acidic in nature. Carboxylic acid also gives this test.
How to detect phenolic functional group?
Any of the following tests can be carried out to detect the phenolic functional group. Scientists use litmus paper to test whether the given solution is acidic or basic. Red litmus paper turns blue while blue litmus paper remains unchanged in the presence of a base. Phenol turns blue litmus paper red. This shows that phenol is acidic in nature.
How to detect carboxylic functional group?
Any of the following tests can be carried out to detect the carboxylic functional group. The carboxylic acid turns blue litmus red. Because the hydroxyl group present in -COOH is far more acidic than in alcohol. The chemical reaction is given below. Note: If the colour of the blue litmus paper changes to red, then a carboxylic group is present.
What is the chemical reaction between carboxylic acid and phenol?
The chemical reaction is given below. Note: This test is used to distinguish between carboxylic acid from phenol. Phenol does not give this test. Carboxylic acid reacts with alcohol in the presence of concentrated sulphuric acid and forms a pleasant smelling ester. This reaction is known as esterification. The chemical reaction is given below.
Past day
Test for Phenolic Group
Scientists use litmus paper to test whether the given solution is acidic or basic. Red litmus paper turns blue while blue litmus paper remains unchanged in the presence of a base. Phenol turns blue litmus paper red. This shows that phenol is acidic in nature. Carboxylic acid also gives this test. lgo algo-sr relsrch richAlgo" data-12a="645fa154335c3">byjus.com › chemistry › test-for-phenolic-groupTest for Phenolic Group - Chemistry Practicals Class 12 - BYJU'S byjus.com › chemistry › test-for-phenolic-group Cached
|
Lab 14: Qualitative Organic Analysis - California State University
functional group, it is recommended that you start with solubility tests, and then conduct Both strong and weak acids (Carboxylic acids and phenols) will be |
|
Identifying an Unknown Compound by Solubility, Functional Group
To verify that a compound has dissolved, add 5 HCl to the NaOH mixture until the solution is acidic to pH paper Look for a precipitate, indicating that the water-soluble salt has converted back into the water-insoluble compound Solubility in NaOH indicates either the carboxylic acid or phenol |
|
Functional group detection - Shivaji College
Test for carboxylic acid group indicates the presence of carboxylic acid group Phthalein test- The phenols having a free para position respond to this test |
|
Lelm108pdf - NCERT
glacial acetic acid Both the above reactions are used as tests for unsaturation 24-04-2018 This is a test that distinguishes carboxylic acids from phenols |
|
Identification of Organic Compound by Organic Qualitative Analysis
Test Observation Inferences (a) Nature i) Solid Carbohydrate, acid , phenol ester, phenol, amines may be present Ester, alcohol and halogen derivatives |
|
LABORATORY 5 DETECTION OF FUNCTIONAL GROUPS IN
Procedure: Add dropwise 1 acetic acid solution of bromine to about 0 5 ml of sample dissolved in acetic acid The test with potassium permanganate by Lehman Ferric chloride reacts with the hydroxyl groups of phenols and phenol |
|
Pdf file
solubility in strong base suggests that it is at least weakly acidic (eg, phenols) test whereby the ester is converted to a hydroxamic acid (HOHN-C=O) which will |
|
Org Chem Manual, 2013pdf
solubility is the desired positive test for acidic or basic functional groups Below is a have the ability to react with weak bases (carboxylic acids) and phenols |
|
Practical identification of organic compoundsdocx - DAV College
The identification of organic compounds by qualitative tests involves a study of Carboxylic acids Amines Ethers Phenols Quinones Aldehydes Anhydrides |
|
Qualitative Test for Phenol - CUTM Courseware
Phenol turns blue litmus paper red This shows that phenol is acidic in nature Carboxylic acid also give this test Compare to carboxylic acid phenol is weakly |