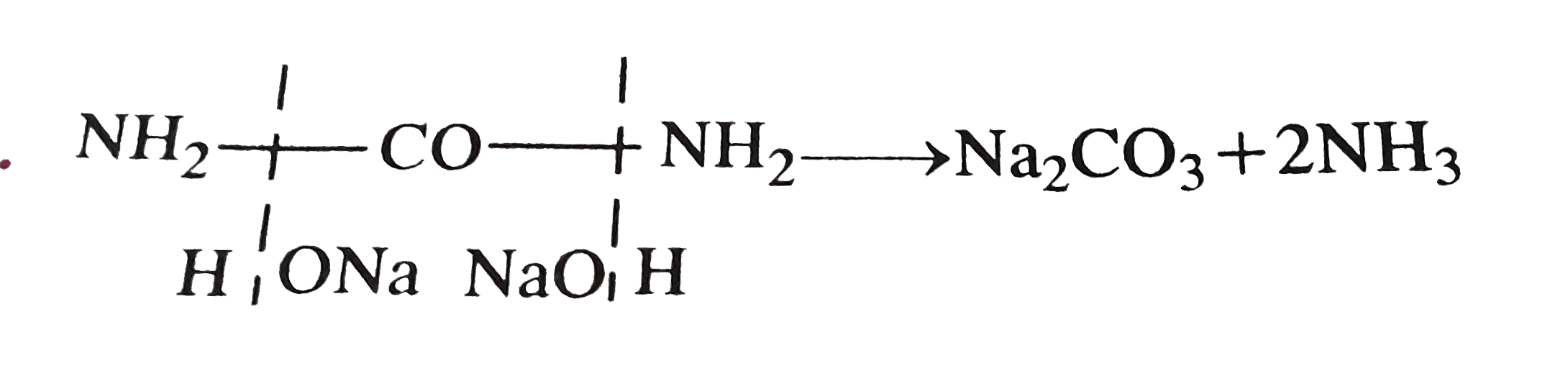the base hydrolysis of benzamide by naoh produces
|
Chapter 5 Carboxylic Acids and Esters
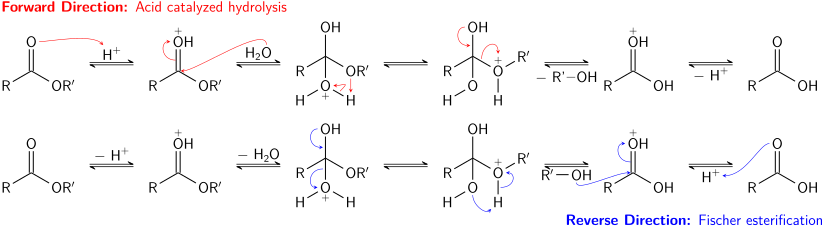
predict the products of ester synthesis and hydrolysis reactions. + NaOH. H2O. O. O carboxylic acid base metal carboxylate. CH3CH2CH2CH2. |
|
Chapter 6 Amines and Amides
Learn the major chemical reactions of amines and amides and learn how to predict the products of amide synthesis and hydrolysis reactions. • Learn some of the |
|
Intermediates in the Reactions of Carboxylic Acid Derivatives. IV
produced and the liquid product was com- between benzamide-O18 and water during the basic hydrolysis of benzamide ... B |
| Selective Hydration of Nitriles to Corresponding Amides in Air with |
|
Separation purification and identification of the components of a
values and recuperation “yields” measurement of physical properties |
|
Synthesis of paracetamol by acetylation
Usually the yields of this reaction |
|
Lab 14: Qualitative Organic Analysis
High MW basic (i.e. amines). Insoluble in. 5% HCl. Soluble in. 5% NaOH phase (the carboxylic acid) when the hydrolysis mixture is acidified. |
|
Reactions of Amines
H-X (proton acid). NaOH amine base ammonium salt. (acidic) Fairly high temperatures often required and yields aren't as good as with acid chlorides. |
|
The von Richter Reaction. V. Evidence that Benzonitrile and
V. Evidence that Benzonitrile and Benzamide ates might be hydrolyzed in the reaction mixtures. ... that -naphthoic acid in 13% yield is produced by. |
|
Hydrolysis of Benzamide - Geocitiesws
Benzamide C6H5CONH2 can be hydrolysed by NaOH forming ammonia and benzoic acid Procedure Weigh about 3g of benzamide Place it in a 250 cm3 (or smaller) conical flask and add to it 50 cm3 of sodium hydroxide Fit the flask with a cork carrying a glass tubing and boil the mixture for about 15 minutes |
How do you go from benzamide to benzoic acid?
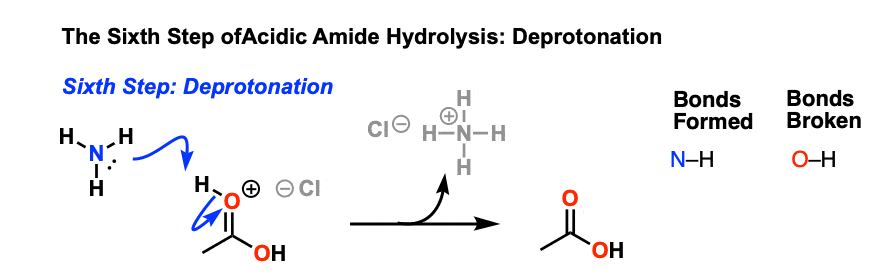
Let's say our goal was to go from benzamide to benzoic acid. We just learned two ways to do that. One way to do that would be to add water and an acid. We get H3O plus and if we heat things up we know that we can hydrolyze our amide that way and give us our benzoic acid, so that's one possibility. That's acid-catalyzed amide hydrolysis.
What is the result of the hydrolysis of a simple amide?
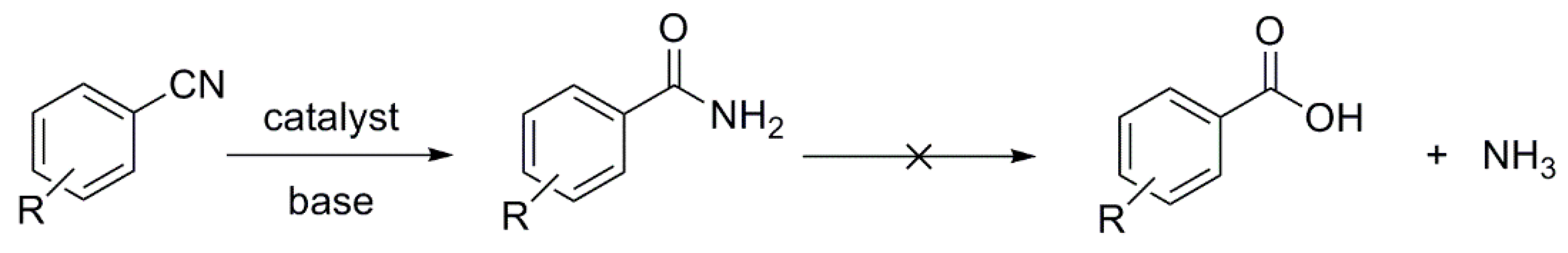
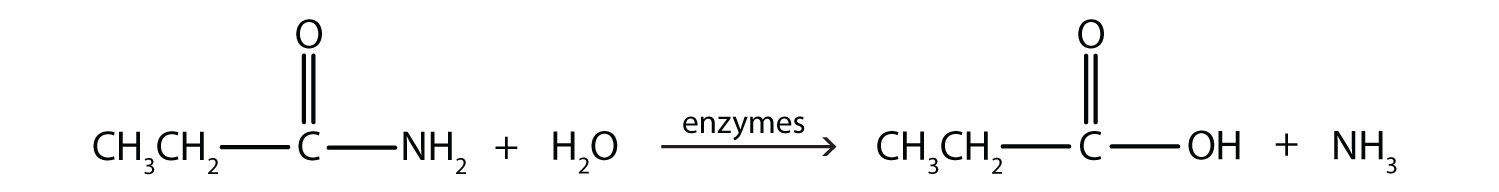
The hydrolysis of a simple amide produces an organic acid and ammonia. Butyramide thus yields butyric acid and ammonia. The hydrolysis of an amide produces an organic acid and ammonia. Benzamide thus yields benzoic acid and ammonia.
How do you hydrolyze amide?
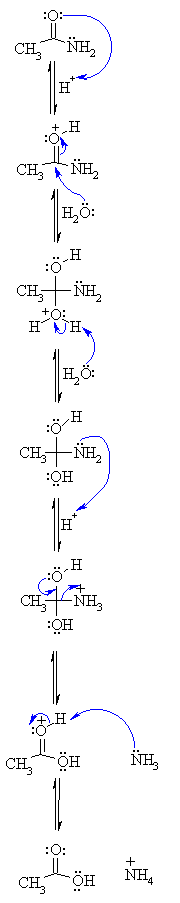
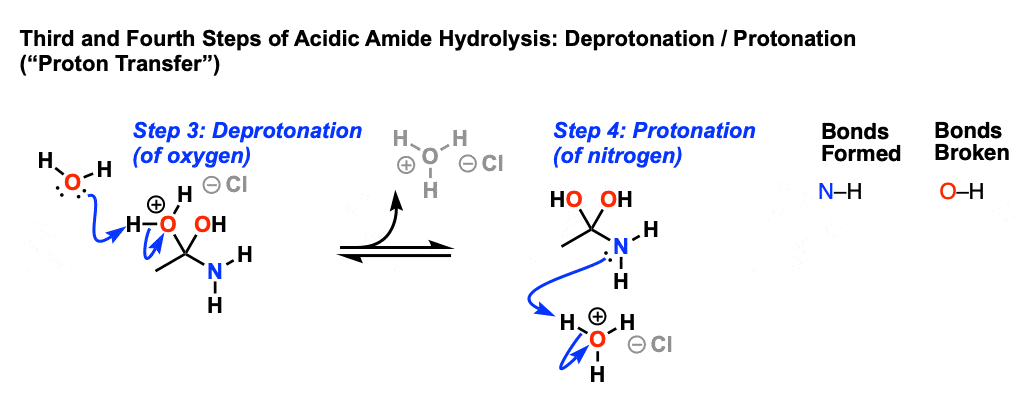
One way to do that would be to add water and an acid. We get H3O plus and if we heat things up we know that we can hydrolyze our amide that way and give us our benzoic acid, so that's one possibility. That's acid-catalyzed amide hydrolysis. Or we could use base.
What is the reaction between a strong base and an acid?
We have a strong base and we have an acid. We're going to have an acid-base reaction. The base is going to take a proton from the acid. Let's say these electrons in red take a proton from the acid, leave these electrons behind. Let's go ahead and draw the product. We would have the conjugate base to a carboxylic acid, which is a carboxylate anion.
|
Benzoic Acid from Ethyl Benzoate by Base Hydrolysis
However, ethyl benzoate is found to react much faster with aqueous sodium hydroxide, the reaction going to completion, to give sodium benzoate (water soluble) |
|
Chapter 13 Carboxylic Acids, Esters, Amines, and Amides
Esters, Amines, and Amides Esterification is the reaction of a carboxylic acid and NaOH O CH 3 —C—O– Na+ + NH 3 base hydrolysis Hydrolysis |
|
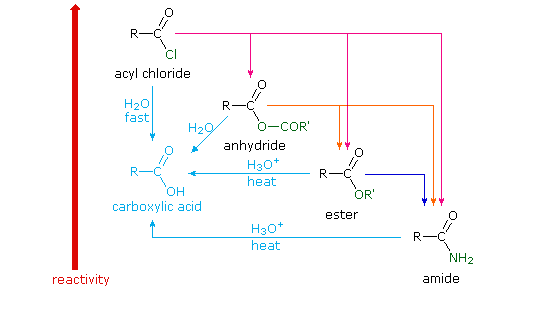
Chapter 21 Reaction Summary (Carboxylic Acid Derivatives)
Reaction of an acid chloride with an alcohol in the presence of a base such In the presence of two equivalents of amine, acid chlorides can be converted to amides Hydrolysis: Esters can be hydrolyzed to carboxylic acids and alcohols in the for this reaction Common acids include H2SO4 and HCl N O H2O NaOH |
|
Reactions of Amines
H-X (proton acid) NaOH amine base ammonium salt (acidic) • Mechanism: Required (protonation) Reaction with Ketones or Aldehydes (Section 18-16,17 and 19-10) R' R Acylation with Carboxylic Acids to From Amides: (Section 20- 12) |
|
Carboxylic acid deri
amide acid chloride acid anhydride |