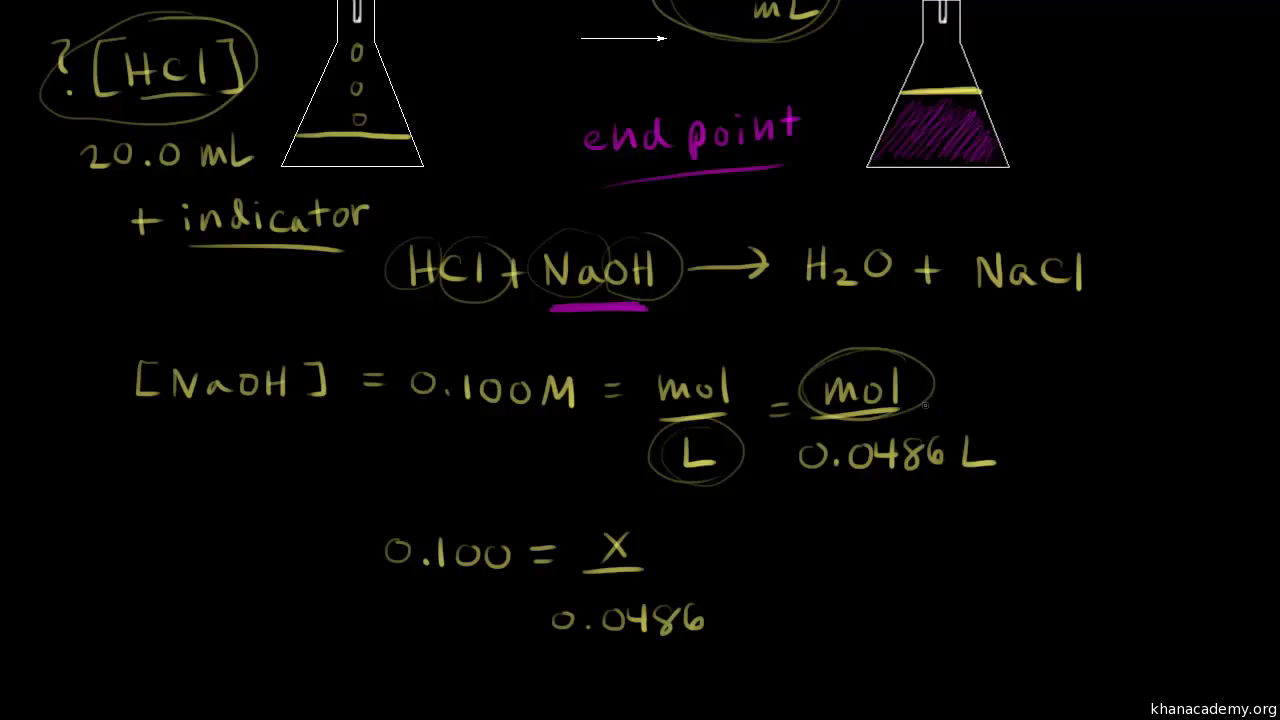the concentration of a solution as determined by titration meaning in hindi
|
TITRIMETRIC ANALYSIS
Determination of the concentration (strength) of a given sodium hydroxide solution by titrating it against a standard solution of oxalic acid. Theory. In the |
|
Determination of Chloride Ion Concentration by Titration (Mohrs
The chromate solution needs to be prepared and used with care as chromate is a known carcinogen. Silver nitrate solution causes staining of skin and fabric ( |
|
1 Determination of calcium by Standardized EDTA Solution
The classic method of determining calcium and other suitable cations is titration with a standardized solution of ethylenediaminetetraacetic acid (EDTA). |
|
TITRIMETRICANALYSIS (REDOX REACTIONS)
redox titrations there is a change in oxidation potential of the system. To determine the concentration/molarity of KMnO4 solution by titrating it ... |
|
Determination of Chloride Ion Concentration by Titration (Volhards
Silver nitrate solution causes staining of skin and fabric (chemical burns). Any spills should be rinsed with water immediately. Concentrated nitric acid is |
|
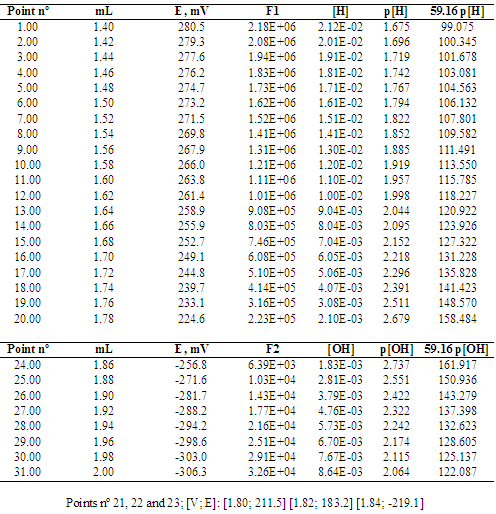
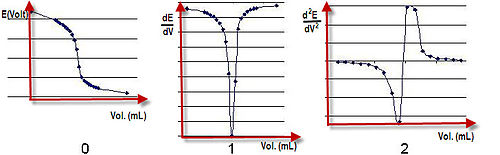
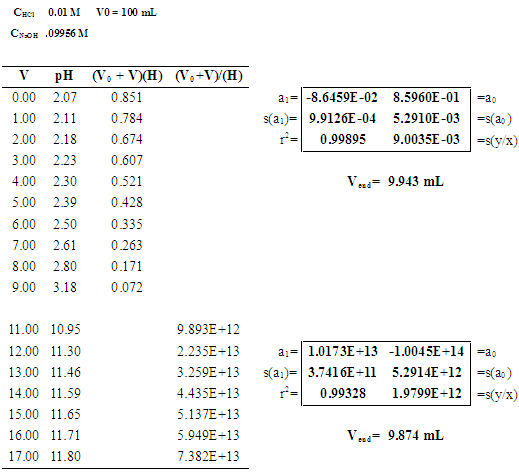
PH titration
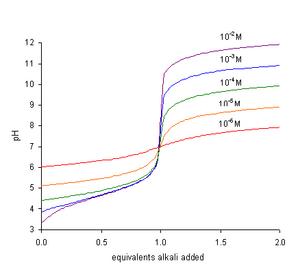
From the resulting titration curves you will determine the concentrations of the acidic solutions as well as the acid-ionization constant of a weak acid. |
|
Determination of Total Calcium and Magnesium Ion Concentration
For the titration the sample solution containing the calcium and magnesium ions is reacted with an excess of EDTA. The indicator is added and remains blue as |
|
DETERMINATION OF PEROXIDE VALUE
The concentration of peroxides is indicative of Add 1 ml of 1.5% starch solution as indicator and continue the titration until dark. |
|
Bellevue College
This mean one cannot of any sodium hydroxide solution is by titration. Determining ... A titration is a procedure for determining the concentration of a. |
|
GENERAL TESTS PROCESSES AND APPARATUS
of arsenazo III TS with a measuring pipet and titrate ;2.50= with 0.005 mol/L sulfuric acid VS. Perfrom the test with the blank solution in the same manner |
|
THEORY OF QUANTITATIVE ANALYSIS - Hindi Mahavidyalaya
The analytical method wherein the concentration of a substance in a solution is estimated by adding exactly the same number of equivalents of another substance present in a solution of known concentration is called volumetric analysis Titrant or Titrator is a standard solution whose concentration and volume are known |
|
62 General principles and terms of titration processes
The titrant solution containing the active agent with which a titration is made is standardized i e the concentration of the active agent is determined usually by titration with a standard solution of accurately known concentration Standard solutions are prepared using standard substances in one of several ways |
How does titration determine concentration?
Titration is a very useful laboratory technique in which one solution is used to analyse another solution. One of the solutions is a standard solution of known concentration and is delivered from a burette.
Why is titration a primary or secondary standard?
The solution called the titrant must satisfy the necessary requirements to be a primary or secondary standard. In a broad sense, titration is a technique to determine the concentration of an unknown solution.
What is a titrant solution?
The titrant solution, containing the active agent with which a titration is made, is standardized , i.e., the concentration of the active agent is determined, usually by titration with a standard solution of accurately known concentration. Standard solutions are prepared using standard substances in one of several ways.
How do you titrate an acid solution with a base solution?
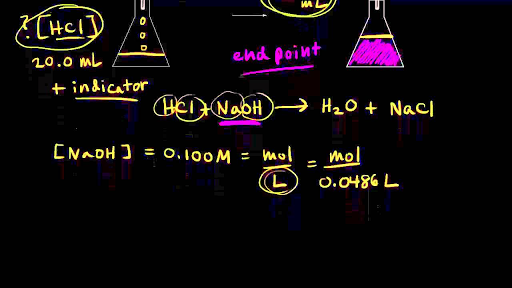
Suppose you are titrating an acid solution with a base solution of known concentration. To calculate the concentration of the acid solution, use three steps. 1. Use the delivered volume of base to reach the endpoint and the known concentration of the base solution to find the moles of base used. 2.
|
TITRIMETRIC ANALYSIS - NCERT
be determined about it later in this unit in the titration of sodium hydroxide with oxalic acid concentration of solution is expressed in terms of molarity It is |
|
N by Titration (Mohrs Method)
Any spills should be rinsed with water immediately Introduction This method determines the chloride ion concentration of a solution by titration with silver nitrate |
|
Determination of Chloride Ion Concentration by Titration (Volhard)
Silver nitrate solution causes staining of skin and This method uses a back titration with potassium The concentration of chloride ions is determined by |
|
Calcium Analysis by EDTA Titration
Water hardness can be readily determined by titration with the chelating agent EDTA In a titration to establish the concentration of a metal ion, the In this experiment you will standardize a solution of EDTA by titration against a standard |
|
Analytical Chemistry - Mettler Toledo
How to get the best titration results reagent (titrant) of known concentration which is added to the sample A well-known example is the titration of The amounts of water generally determined via coulometric Karl Fischer titration are smaller dilutes the sample again, making the solution less opaque The profile of the |
|
Nitrogen Determination by Kjeldahl Method - PanReac AppliChem
14 jan 2018 · Titration Organic nitrogen is converted into NH4 + NH3 is distilled and ammonia is quantitatively captured by the boric acid solution forming solvated The concentration of the captured ammonium ions can be determined |
|
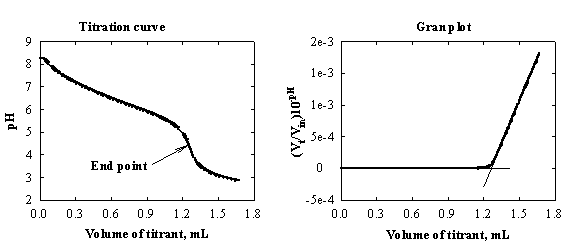
624 Conductometric Titrations
conductivity with the result that conductivity of the solution varies during the course cation to be determined, or vice versa, the more acute is the angle of titration curve know the conductance is a non-specific property, concentration of other |
|
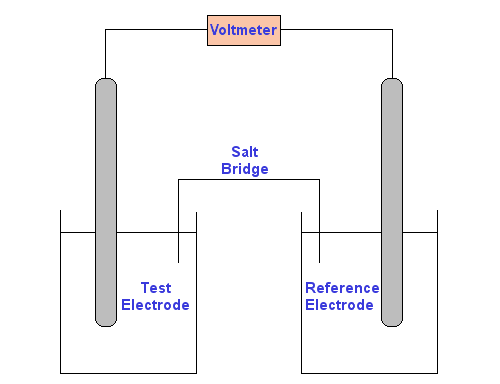
Titration Theory and Practice - IGZ Instruments
concentration of a product, a species or a chemical function in order to ensure the the exact quantity of the analyte can be determined by simple calculation reference electrode junction potential altering the pH of the solution compared to |









/beakers-of-acidic-and-basic-solutions-after-litmus-indicator-has-been-added-litmus-changes-its-colour-depending-on-the-ph-of-the-solution-683739931-586030ee3df78ce2c3550a7e.jpg)




/chemistry-research-172591498-5bf4202046e0fb00516ad4ce.jpg)