theoretical ethanol yield calculator
|
Quantifying Actual and Theoretical Ethanol Yields for Switchgrass
Aug 21 2010 The theoretical ethanol yield calculator used by Lorenz et al. [13] does not account for variability in pretreatment effects |
|
126 J/(K mol ) ethanol ethene water g g g H S ? ?
Then they were asked to calculate the theoretical yield in moles if the reaction proceeded to completion. In part (b) students were asked to use their |
|
Estimation of Ethanol Yield in Corn Mash Fermentations Using Mass
An equation to estimate the ethanol yield by mass of ash in fer- mentation was generated. evaluate ethanol yield such as theoretical yield and starch. |
|
Calculation of Ethanol Production from Fermentation
If 0.021 moles of CO2 are produced how much ethanol was produced? Glucose (a 6 carbon sugar) yields two ethanol molecules and two CO2 molecules. For every |
|
Accounting for all Sugars Produced during Integrated Production of
ethanol and highlights techniques for accurate yield calculations. the ethanol produced from glucose assuming a 95% theoretical metabolic yield from ... |
|
EVALUATION OF SWITCHGRASS VARIETY COMPOSITION AND
May 9 2009 'Theoretical Ethanol Yield Calculator'. Biomass yield data of eight cultivars in a twenty- year (1989-2008) study was analyzed using ... |
|
BIOPROCESS ENGINEERING
Calculate the yield coefficients Yys (g dw cell/g substrate) Estimate the theoretical growth and product yield coefficients for ethanol fermentation by. |
|
Topic 6: Common Lab Calculations
the theoretical yield of your reactions and the percent yield of your reactions once you have isolated the product. Calculating Grams from Molecular Weight. |
|
Analysis of Lifecycle Water Requirements of Transportation Fuels
Estimate grain and cob yields . for ethanol from corn grain and corn cob (crop residue) based on default or ... "Theoretical Ethanol Yield Calculator. |
|
Supporting Information for ?The Water Footprint of Biofuels: A Drink
The Feasibility of Ethanol Production from Sugar cane in the U.S. [7]. Switchgrass. 311. 80% of theoretical ethanol yield calculator DOE [6]. Poplar tree. |
|
Ethanol from Fermentation - College of Engineering
The amount of glucose used for cellular energy can be estimated by a simple mass balance equation and approximate stoichiometric equations relating the amount glucose consumed to the amount of ethanol or biomass produced: ?S = ?S energy + ?S biomass + ?S |
|
The Fermentation of Sugar and the Isolation of Ethanol by
1 Calculate the theoretical yield of ethanol using sugar(51 5g) as the limiting reagent 2 Steps to calculate the percentage ethanol o Graph refractive index vs ethanol and find a best fit line The following web site has RI vs information http://www thewhiskystore de/experts/alcohol htm The data was not linear |
| Le |
Overview
This article explains the concepts of excess reagents and limiting reagents in chemical reactions. It also covers how to calculate theoretical yields and actual yields from known amounts of reactants. The article provides examples on how to determine the amount of iodide ions present in a solution using AgNO3 as an excess or limiting reagent.
Excess & Limiting
Reactants not completely used up are called excess reagents, and the reactant that completely reacts is called the limiting reagent. Theoretical yields can be calculated from reaction stoichiometry. Actual yield is usually less than theoretical yield due to loss in process or inefficiency of chemical reaction.
Calculate Yields
Estimate theoretical and percentage yields, evaluate actual yields from known amounts of reactants, calculate theoretical yields of products formed in reactions involving limiting reagents.
How do you calculate ethanol yield factor?
Using the ethanol production equation, calculate the theoretical maximum yield factor, YP/S = g/L ethanol / g/L glucose, if 20 g/L glucose was consumed. Proper attire is worn (long pants and closed-toe shoes). Food and drinks are stored and consumed outside the laboratory. Lab coat and safety glasses are worn.
How do you calculate the theoretical yield of a limiting reactant?
Based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. Calculate the percent yield by dividing the actual yield by the theoretical yield and multiplying by 100. A From the formulas given for the reactants and the products, we see that the chemical equation is balanced as written.
What is the ratio of actual yield to theoretical yield?
The ratio of actual yield to theoretical yield expressed in percentage is called the percentage yield. Chemical reaction equations give the ideal stoichiometric relationship among reactants and products. Thus, the theoretical yield can be calculated from reaction stoichiometry.
What is the theoretical yield of 1.2 metric tons of hydrogen gas?
(2) CO ( g) + 2 H 2 ( g) ? CH 3 OH ( l) (3) 28.0 4.0 32.0 ( stoichiometric masses in g, kg, or tons) Thus, the theoretical yield from 1.2 metric tons (1.2x10 6 g) of hydrogen gas is 9.6 tons.
|
Quantifying Actual and Theoretical Ethanol Yields for - CORE
21 août 2010 · theoretical ethanol yield calculator used by Lorenz et al [13] does not account for variability in pretreatment effects, enzymatic hydrolysis, or |
|
Calculation of Ethanol Production from Fermentation
(0 5 liters) • (0 042 mole/liter) = 0 021 moles of CO2 If 0 021 moles of CO2 are produced, how much ethanol was produced? Glucose (a 6 carbon sugar) yields two |
|
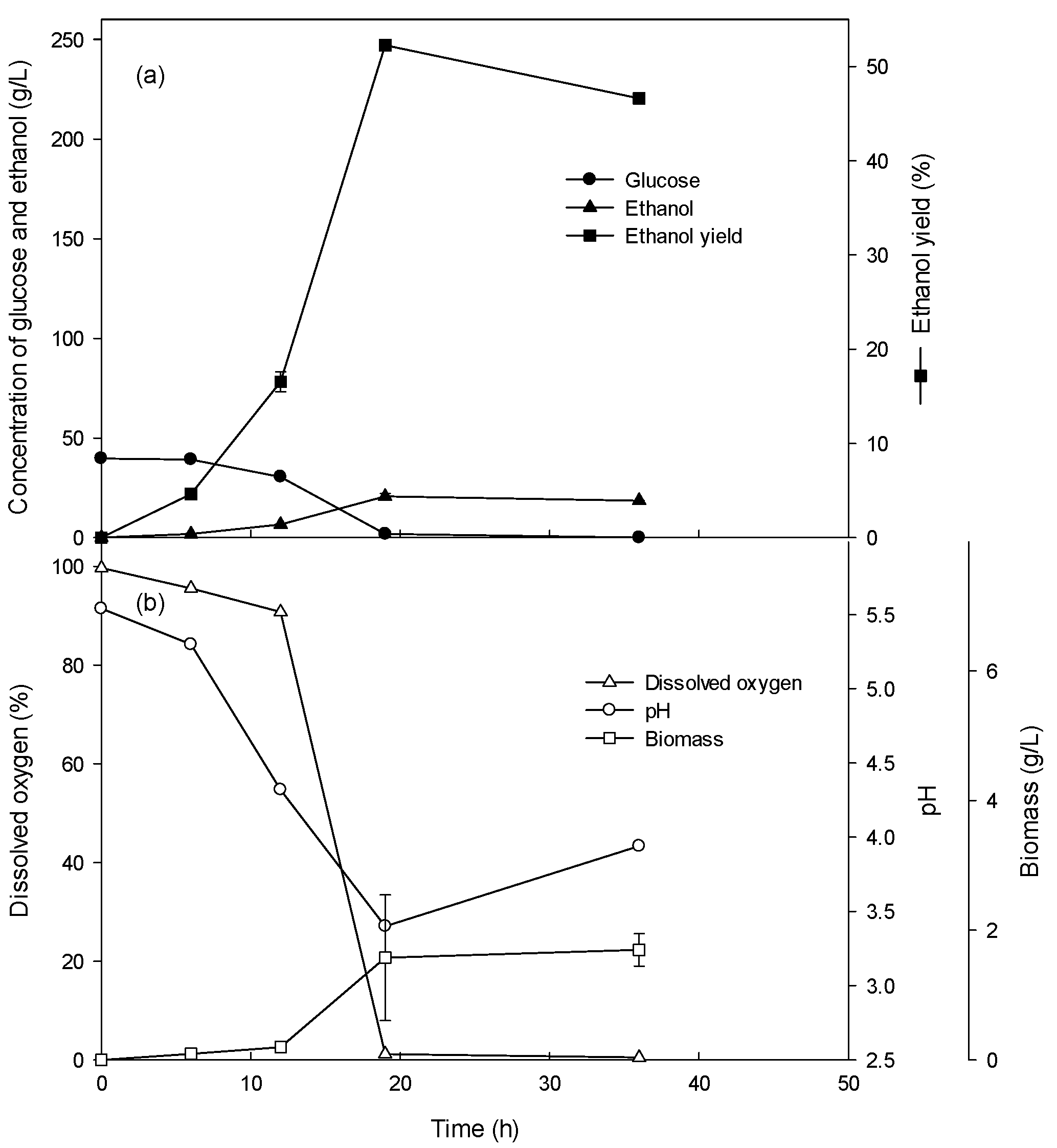
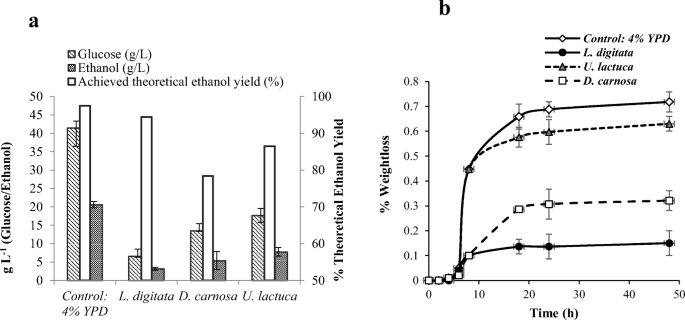
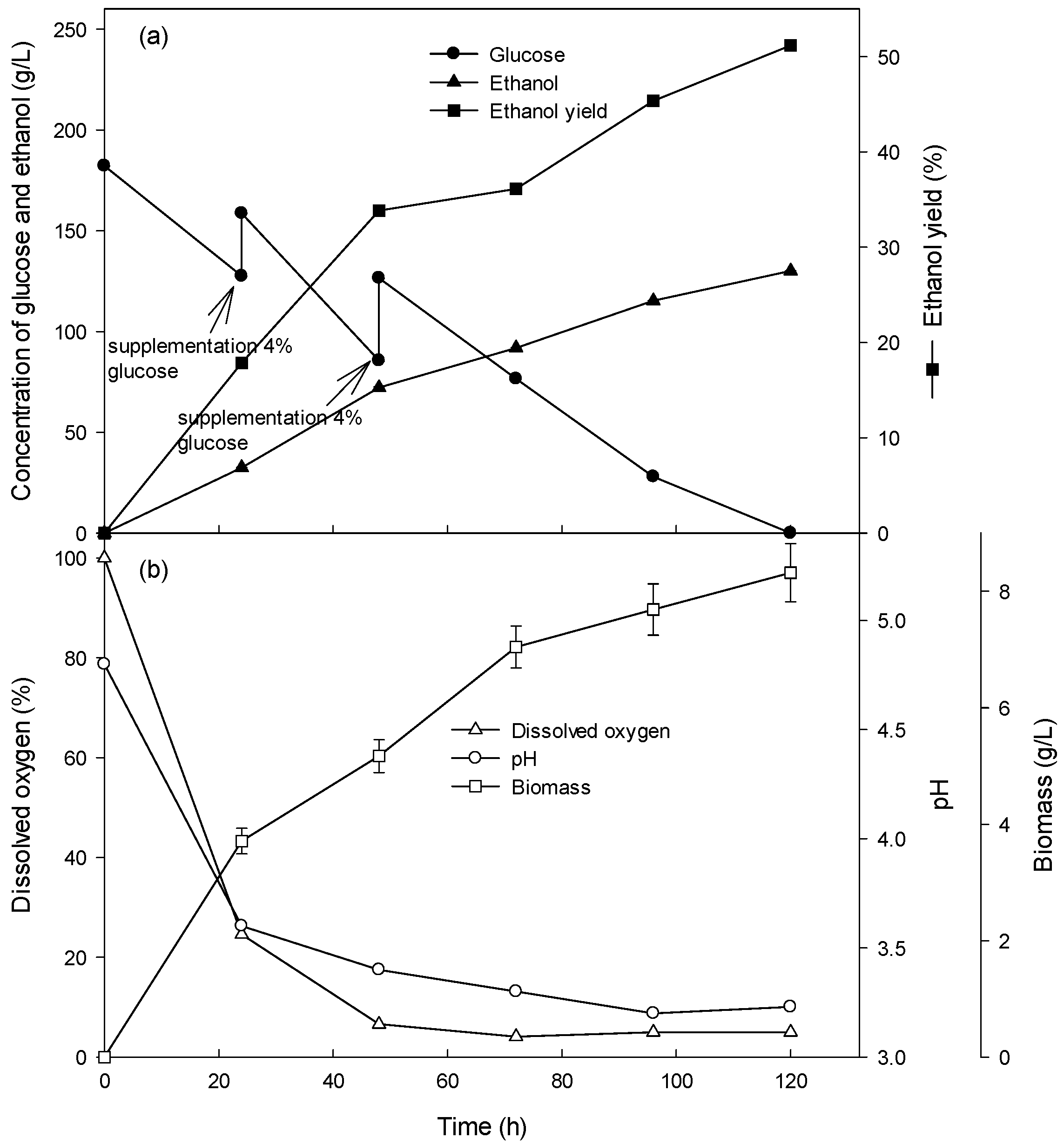
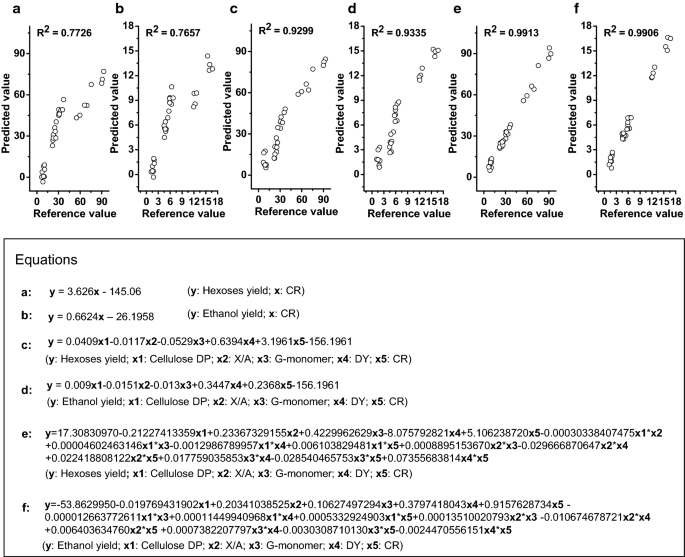
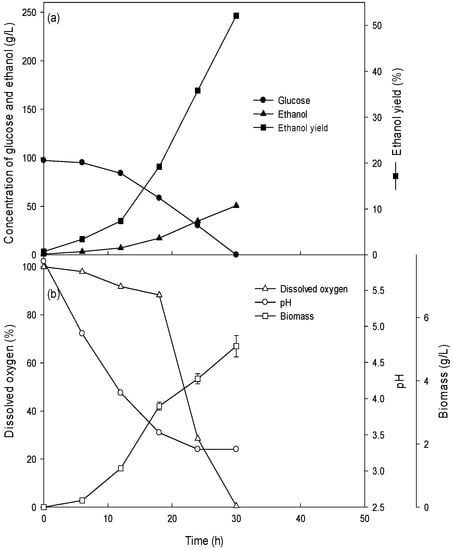
Novel ethanol production using biomass - Research Square
1 Ethanol fermentation yield is expressed as a percent of theoretical yield (Y T), per unit of sugar (g/g) was used to calculate the percent theoretical yield |
|
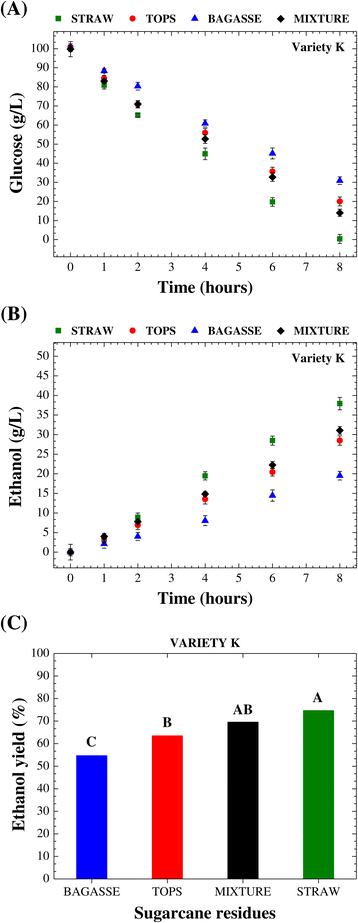
This article appeared in a journal published by Elsevier The
A modified method for calculating practical ethanol yield at high feedstock may reach 30–40 by the weight percentage (grams sol- ids in 100 g of the total |
|
RedalycBIOETHANOL POTENTIAL FROM HIGH DENSITY SHORT
24 mar 2017 · densities The average theoretical ethanol yield at 48 months reached 395 9 L t-1 for dry wood basis; this value was used to calculate the |