La theorie d'Arrhenius
|
Development of the theory of electrolytic dissociation*
46 1903 S A ARRHENIUS applies This dissimilarity between the two sister-sciences has frequently led to differences of opinion in physical chemistry That discontinuous changes and multiple proportions also occur in physics has been assumed in many cases which on closer examination disproved this assumption |
|
Chapter The Arrhenius Acid and Base Theory
The concept of acids and bases have been defined many times in different ways Several scientists put various definitions to characterize the acids and bases in which some of the concepts are quite narrow and some are comprehensive Acids and bases are existing everywhere in our daily life Every liquid except water that we used having acid and ba |
|
THE THEORY OF ACIDS AND BASES
The Water or Arrhenius Theory was widely accepted up to the early years of this century It defines an acid as a hydrogen compound ionizing in water to give hydrogen ions and a base as a hy |
|
Lecture 22: The Arrhenius Equation and reaction mechanisms
Learn about two theories developed to explain kinetics: collision and transition state theory Learn about how the rate law for a reaction is created from the reaction mechanism Look at some famous catalysts First a summary of the differential and integrated rates laws from the first kinetics lectures: |
|
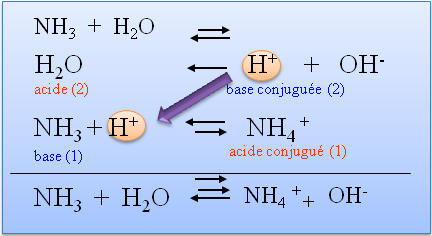
I La théorie d’Arrhénius sur les acides et les bases
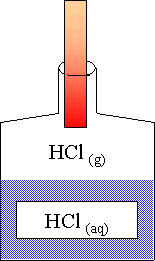
Svante Arrhénius un chimiste suédois a développé une théorie en 1887 pour expliquer les acides et les bases Le principe de cette théorie est qu’il définit les acides et les bases selon leur structure et selon les ions qu’ils produisent lorsque dissous dans l’eau selon les équations ci-dessous : HA H2 O H+ + A HCl(g) H2 |
What is the difference between Arrhenius theory and Bronsted-Lowry theory?
According to Arrhenius theory, hydrochloric acid is an acid which gives hydro- gen ions in water but according to Bronsted-Lowry theory, hydrochloric acid is an acid because it donates a proton to the water molecule. By observing both concepts, water is acting as a base. So, we can see here that both theories are very similar to each other.
What did Svante Arrhenius say about ionization?
Swedish Svante Arrhenius, in 1884 proposed the concept of acid and base based on the theory of ionization. According to Arrhenius, the acids are the hydrogen- containing compounds which give H+ ions or protons on dissociation in water and bases are the hydroxide compounds which give OH ions on dissociation in water.
What is Arrhenius theory of electrolytic dissociation?
The Theory of Electrolytic Dissociation – Subsequent to Arrhenius The fundamental idea proposed by Arrhenius in 1887, that ionic compounds dis- sociate spontaneously into their ions when dissolved in water, still holds today but some of the details have changed to accommodate anomalies.
What is Arrhenius theory?
The Water or Arrhenius Theory was widely accepted up to the early years of this century. It defines an acid as a hydrogen compound ionizing in water to give hydrogen ions, and a base as a hy
1. Introduction
The concept of acids and bases have been defined many times in different ways. Several scientists put various definitions to characterize the acids and bases in which some of the concepts are quite narrow and some are comprehensive. Acids and bases are existing everywhere in our daily life. Every liquid except water, that we used having acid and ba
1.2 Factors affecting acidic strength
The strength of acids and bases depends on following factors: Polarity of the molecule and strength of H▬A bond Electro negativity Size cdn.intechopen.com
1.2.1 Polarity of the molecule and strength of H▬A bond
As the polarity of the molecule increases, the electron density will get away from hydrogen atom and it becomes H+(proton). The greater is the positive charge on the hydrogen atom, H▬A bond will become weaker, lesser is the energy required to break it. Then, the proton will easily dissociate in the solution. Hence, it will be the strong acid [1]. cdn.intechopen.com
1.2.1.1 Key points
The priority should be given to the polarity of H A bond, when we compare the ▬ acidic strength of elements in the same row. But when we compare the acidic strength of elements of same group of periodic table, then priority is given to strength of H▬A bond. cdn.intechopen.com
1.3 Bases
Bases are those substances which have bitter taste, odorless, turn red litmus blue, having pH more than 7 and becomes less alkaline when react with acid. These are violent and less reactive than acids. For example, NaOH (Sodium hydroxide), LiOH (Lithium hydroxide), KOH (potassium hydroxide), etc. These are the general properties of acids or bases,
2.1 Neutralization reaction
When Arrhenius acid and Arrhenius base reacts, salt and water is formed as product, the reaction is known as neutralization reaction. For example: The acids which are completely ionized in aqueous solution, is termed as strong acids such as HCl, HNO3, H2SO4, etc. Hydrochloric acid is a strong acid. When it dissociates into water, hydronium ion and
3. Utility of Arrhenius concept
This theory explains many phenomena like strength of acids and bases, salt hydrolysis and neutralization. cdn.intechopen.com
6. Amphoteric nature of water
The word amphoteric is derived from Greek word“amphi ” that means both (acid and base). Amphoteric substances are those that has potential to act either as an acid or base. For example: H2O (water) [3]. On dissociation, it ionizes into H+and OH (hydroxide) ion. The presence of H+ indicates an acid and the presence of OH ion indicates a base. Since,
7. Advantages of Arrhenius theory
This theory is used to explains: Strength of acid and bases The strength of Arrhenius acid and Arrhenius base can be determined by the extent to which it dissociate to give H+ ion or hydroxide ion [5]. The properties of acids and bases in aqueous medium. Neutralization of acid by reaction with base cdn.intechopen.com
8. Limitation of Arrhenius acid-base theory
This theory is very limited, out of three theories. According to this theory, the solution medium should be aqueous and acid should produce hydrogen ion (H+) or base should produce hydroxide ion (OH ) on dissociation with water. Hence, the substance is regarded as Arrhenius acid or Arrhenius base when it is dissolved in water. For example, HNO3 is
9. Bronsted-Lowry theory
We have been previously learned an Arrhenius acid-base theory which provided a good start towards the acid-base chemistry but it has certain limitations and problems. After this theory, a Danish chemist, named Johannes Nicolaus Bronsted and British scientist, Thomas Martin Lowry proposed a different definition of acid-base that based on the abiliti
9.2 Examples of Bronsted-Lowry acids and bases
In this reaction, the nitric acid donates a proton to the water, therefore it act as a Bronsted-Lowry acid. Since, water accepts a proton from nitric acid, so it is act as Bronsted-Lowry base. In this reaction, the arrow is drawn only to the right side which means that reaction highly favours the formation of products. In this reaction, the water i
10. Relation between Arrhenius theory and Bronsted-Lowry theory
These two theories are not against to each other in any way, in fact Bronsted-Lowry theory is advance to the Arrhenius theory. According to the Arrhenius theory, a substance which produces hydrogen ion in water, called acid. A substance which produces hydroxide ion in water, called base. According to Bronsted-Lowry theory, an acid is proton donor a
11.1 Lewis acid
According to this theory, an acid is a substance which has capability to accept the non-bonding pair of electrons, called Lewis acid. They are sometimes referred as electron deficient species or electrophile. cdn.intechopen.com
11.2 Lewis base
A base is a substance which has capability to donate the electrons, called Lewis base. They are sometimes referred as electron rich species or Nucleophile. cdn.intechopen.com
11.2.1 Lewis base: characteristics
Lewis base-electron-pair donor All metal anions (F , Cl , Br , I ) are Lewis base because they have ability to donate the electron but all Lewis bases are not anions. The ion, molecule or an atom which having a lone pair of electrons, are also considered as Lewis base. The electron-rich system is also considered as Lewis bases, for example, п benze
11.3 Example of Lewis acid-base
A simplest example of Lewis acid-base is shown by a chemical reaction: In this reaction, chloride ion acts as Lewis base because it has lone pairs of electrons and sodium ion has positive charge, so it acts as Lewis acid. cdn.intechopen.com
11.4 Neutralization reaction between Lewis acid and Lewis base
When a Lewis acid reacts with a Lewis base, then a Lewis acid-base reaction occurs in which the molecule which act as Lewis base donate its electron pair into the empty orbital of an acid, forms Lewis acid-base adduct as shown in Figure 2. The adduct formed contains a covalent coordinate bond between Lewis acid and Lewis base. The above explanation
11.5 Limitations of Lewis acid-base theory
This theory is not able to explain that why all acid-base reactions do not involve the covalent coordination bond. This theory is also unable to explain the behavior of some acids like hydrogen chloride (HCl) and sulfuric acid (H2SO4) because they do not form the covalent coordination bond with bases. Hence, they are not considered as Lewis acids.
13. Conclusion
Acids and bases are very important for modern society and in our daily lives. They exist everywhere in our body and in our surroundings. The theory that has been described in this chapter has given us all the basic information of acids and bases. In this chapter, we have discussed all the three basic theory of acid-base chemistry-Arrhenius theory,
Acknowledgements
am eternally grateful and beholden to my family. My mother Mrs. Suman Munjal, Father Mr. Bhim Sain Munjal and sister Mrs. Shweta Java for strengthening me with the opportunities and experiences which enabled me in reaching these heights. The reason behind this success is their selfless encouragement that helped me explore new dimensions in my life
Declaration
I Shikha Munjal undersigned solemnly declare that all the information submitted by me in this chapter is correct, true and valid. cdn.intechopen.com
|
I. La théorie dArrhénius sur les acides et les bases II. La théorie de
) en solution. Arrhénius a défini une base ainsi : une base est une substance dissous dans l'eau et se dissocie en ion hydroxyde (OH. |
|
Comprendre la relation entre les concepts dArrhénius de Bronsted
8 juin 2019 d'Arrhénius de Bronsted-Lowry et de Lewis. Article Understanding the Relationship Among Arrhenius |
|
La tonométrie différentielle des solutions et la théorie dArrhénius
On sait que d'après la théorie d'Arrhénius |
|
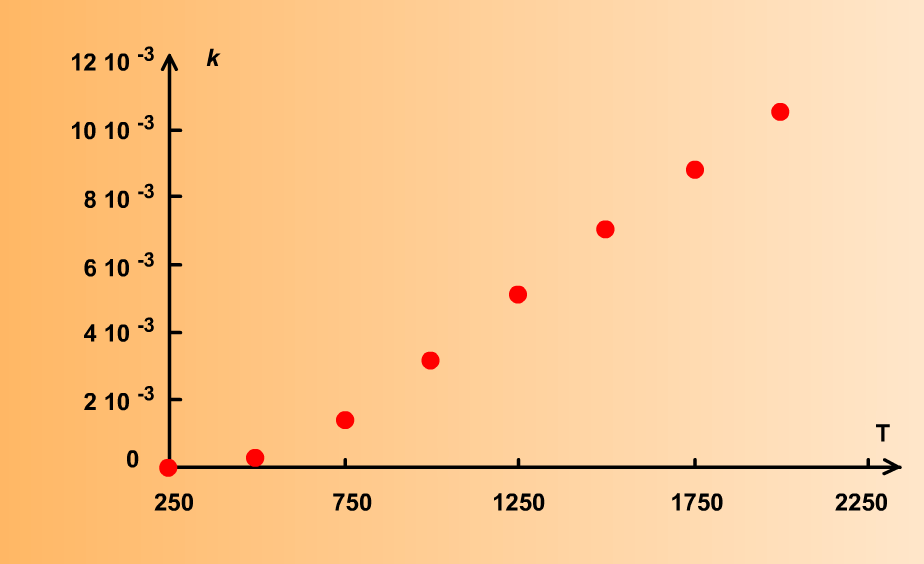
La loi dArrhenius
avec la théorie des collisions et celle du complexe activé. » Le capitaine Tomacz referme le tome 1 (AA-BE) de l'encyclopédie d'un geste qui trahit sa |
|
Les acides et bases en solution aqueuse.
Face aux insuffisances de la théorie d'Arrhenius on a tenté de généraliser les notions d'acide et de base. En 1923 |
|
1. Selon la théorie dArrhenius : 2. Selon la théorie de Brønsted et
Selon la théorie d'Arrhenius : a) Écrire l'équation de la réaction de dissolution dans l'eau de l'acide nitrique de formule HNO3(?) |
|
Jacques GRINEVALD - LEFFET DE SERRE ET LA CIVILISATION
23 juin 2011 la théorie d'Hogbom-Arrhénius car la théorie du cycle du CO2 sur laquelle s'appuie Arrhénius est explicitement celle de son collègue ... |
|
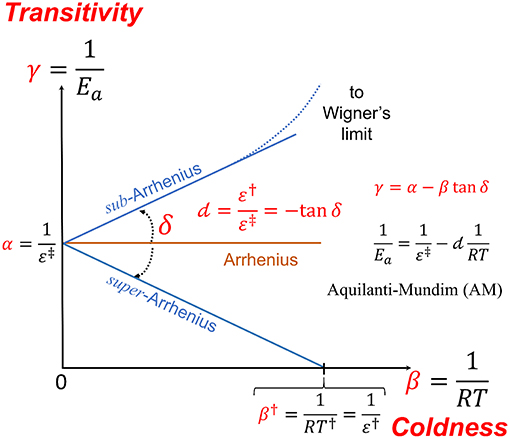
Théorie de létat de transition
Théorie de l'état de transition. De la formule d'Arrhénius au calcul du facteur de fréquence I D'Arrhénius à la faillite de la théorie des collisions. |
|
Etude expérimentale et numérique de la décomposition thermique
La justification de la forme de la loi d'Arrhenius pour modéliser la cinétique des réactions de pyrolyse peut être abordée avec la théorie des états de |
|
Georges Lemaître et la neutralité métaphysique du Big Bang
24 oct. 2020 que l'entendait Georges Lemaître : « cette théorie [la cosmogonie physique du ... satisfait de la théorie d'Arrhénius. |
| I La théorie d'Arrhénius sur les acides et les bases |
| Évolution historique des concepts d'acide et de base |
| 2 Selon la théorie de Brønsted et Lowry - jvince |
| La loi d'Arrhenius - Érudit |
| Les reactions en chimie organique |
| IIDéfinitions des acides et des bases |
| Les acides et bases en solution aqueuse - Groupe Transition |
| La formule deARRHENIUS |
| Théorie de l'état de transition |
| I La théorie d’Arrhénius sur les acides et les bases |
C'est quoi une base selon Arrhenius ?
Quelles sont les insuffisances de la théorie d'Arrhenius ?
. La définition d'une base est liée à celle d'un acide (transfert de proton) ; l'ion OH? ne joue plus de rôle particulier, il devient simplement une base parmi d'autres.
Comment vérifier la loi d'Arrhenius ?
C'est quoi les acides et les bases ?
. Lorsqu'un acide perd un proton, il se transforme en un composé qui peut accepter un proton (une base) : c'est la base conjuguée de l'acide.
|
1 Selon la théorie dArrhenius - jvince
HCℓ (g) est-il un acide ou une base selon Arrhenius ? 2 Selon la théorie de Brønsted et Lowry a) Écrire l'équation de la réaction de l'eau avec l'acide nitrique |
|
IIDéfinitions des acides et des bases
La définition d'Arrhénius ne tient pas compte du fait que c'est souvent un seul des C'est le fondement de la théorie actuelle des phénomènes acido-basiques |
|
Etude expérimentale et numérique de la décomposition thermique
modèle s'appuie sur les théories utilisées dans la phase gazeuse, par conséquent, principes décrits par Arrhenius, à savoir la théorie du « complexe activé » |
|
Arrhénius et Brönsted - physiquesansformule
Acides et bases selon Arrhénius • « Un acide Exemples (selon Arrhénius) : HCl → H+ + Cl- NaOH → Na+ + OH- Les insuffisances de la théorie d' Arrhénius |
|
La loi dArrhenius - Érudit
En cinétique chimique, la loi d'Arrhenius permet de décrire la variation de la avec la théorie des collisions et celle du complexe activé » Le capitaine Tomacz |
|
Votre titre darticle - Revues et Congrès
La théorie des états de transition utilisée pour justifier l'application de la loi d' Arrhenius à la dégradation thermique des solides B Batiota*, T Rogaumea, |
|
LES REACTIONS EN CHIMIE ORGANIQUE
Arrhénius ☞ Brönsted et Lowry ☞ Lewis En effet, les notions d'acidité et de basicité ont considérablement évolué dans le temps, depuis la première théorie |










![PDF] Test of the formal basis of Arrhenius law with heat PDF] Test of the formal basis of Arrhenius law with heat](https://jcmarot.files.wordpress.com/2020/04/theories.png?w\u003d660)





![PDF] Test of the formal basis of Arrhenius law with heat PDF] Test of the formal basis of Arrhenius law with heat](https://cdn.kastatic.org/ka-perseus-images/9289d995e072e697609b0480447718f60ea0ad94.png)



![PDF] Test of the formal basis of Arrhenius law with heat PDF] Test of the formal basis of Arrhenius law with heat](https://www.lachimie.net/images/reacti4.gif)


![PDF] Test of the formal basis of Arrhenius law with heat PDF] Test of the formal basis of Arrhenius law with heat](https://upload.wikimedia.org/wikipedia/commons/b/b3/AceticAcid012.jpg)