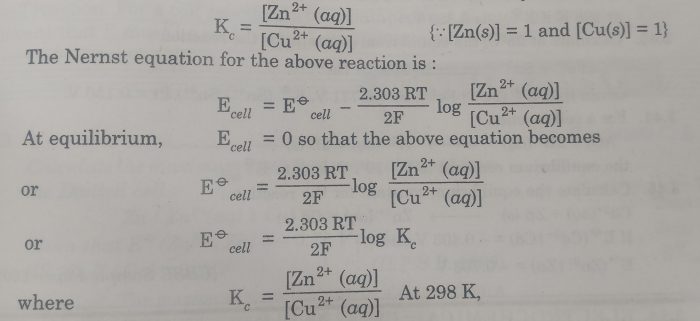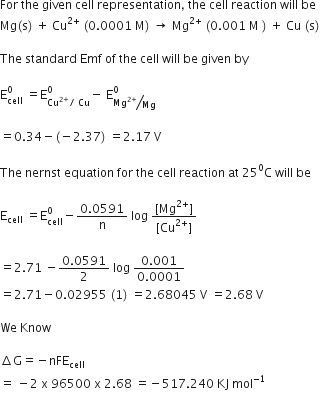all formulas of electrochemistry class 12
What is the difference between electrochemistry and electrolysis?
Electrochemistry: A field of chemistry that focuses on the interchange between electrical and chemical energy Electricity: Flow of electrons over a wire that is affected by the presence and flow of electric charge. Electrolysis: The decomposition of a substance by means of electric current.
What is electron chemistry?
Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as an oxidation-reduction ("redox") reaction.
What is the simplest form of an electrochemical cell?
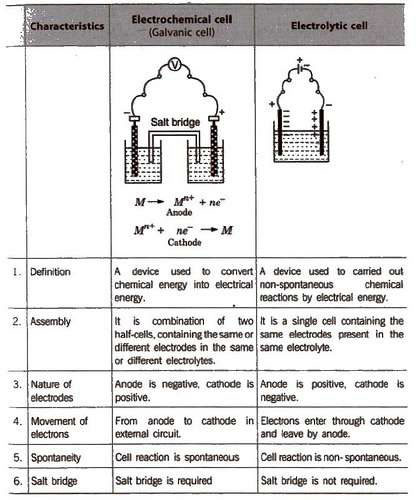
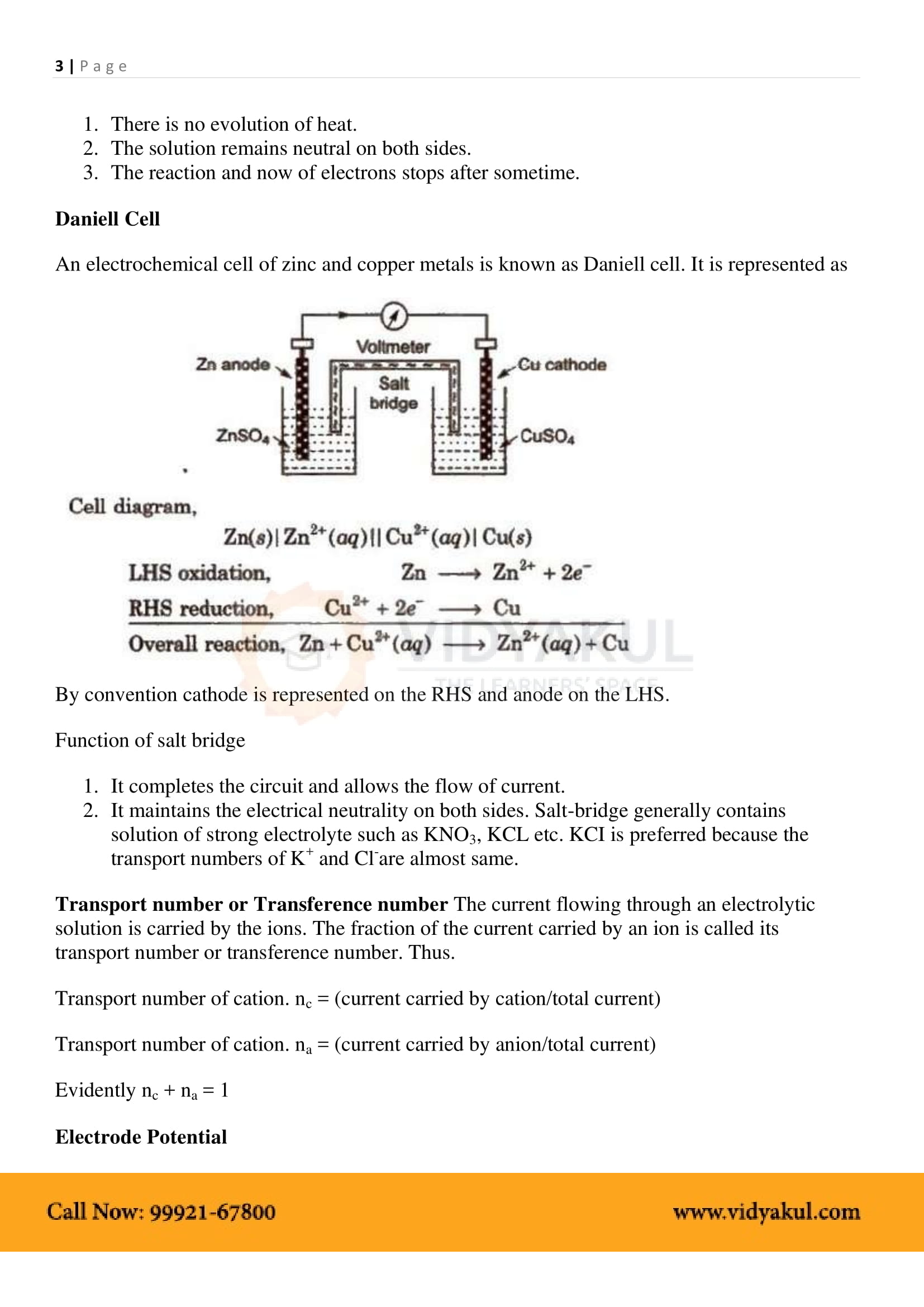
The simplest form of the electrochemical cell is Daniel Cell. The reaction that occurs at the two electrodes are as follows: At cathode: Cu 2+ (aq) + 2e – ⟶ Cu (s). When a metal is placed in a solution of its ions, the metal acquires either a positive or negative charge with respect to the solution.
|
Formulae For CHEMICAL KINETICS
It can be experimentally determined. XII Chemistry. CHAPTER 4 - CHEMICAL KINETICS. If rate law expression for a reaction is. Rate = k |
|
ELECTROCHEMISTRY ENGINEERING CHEMISTRY B.Tech 1 year
02-Apr-2020 All the plates are separated from adjacent plates by insulators like wood strips glass fiber etc. The entire combination is immersed in ... |
|
Chemistry Formula Electrochemistry
Chemistry Notes for class 12 Chapter 3. Electrochemistry. Electrochemistry is The conductivity of all the ions produced when 1 mole of an electrolyte is ... |
|
Chapter 18: Electrochemistry
all other half-cells. Page 9. Reference half-cell : standard ... e.g. A constant current is passed through an electrolytic cell containing molten MgCl2 for 12 h. |
|
Lech103.pdf
Species IO- is called as an intermediate since it is formed during the course of the reaction but not in the overall balanced equation. The first step |
|
Revision Notes Class 12 Chemistry Chapter 3 – Electrochemistry
For this equation we take oxidation potential of anode and reduction potential of cathode. Since anode is put on left and cathode on right it follows therefore |
|
Electrochemistr ochemistr ochemistry Electrochemistr ochemistr
Sodium and magnesium metals are produced by the electrolysis of their fused chlorides and aluminium is produced (Class XII Unit 6) by all the materials that ... |
|
Unacademy
All redox reactions are exothermic. Why? Concept Ladder. According to classical concept oxidation is an addition of oxygen [or. |
|
Electrochemistry Formula Sheet
Page 12. Gradeup CSIR-NET. Super Subscription. Features: 1. Memory Based Test All the CSIR NET Test Series based on the latest pattern and the trend that ... |
|
Formulae For ELECTROCHEMISTRY
12. κ. Λ m x1000. = C. Remember: Unit of Λm in above formula is Scm2mol-1. 13. α c m. 0 m. ∧. = ∧. 14. 2 a cα. K = 1-α. XII Chemistry. CHAPTER 3 - |
|
Formulae For ELECTROCHEMISTRY
Unit of ?m in above formula is Scm2mol-1. 13. ? c m. 0 m. ?. = ?. 14. 2 a c?. K = 1-?. XII Chemistry. CHAPTER 3 - ELECTROCHEMISTRY. |
|
Electrochemistry Formula Sheet
Gradeup CSIR-NET. Super Subscription. Features: 1. Memory Based Test Series of the actual exam paper. 2. All the CSIR NET Test Series based on the latest |
|
Electrochemistry Electrochemistry
In Class XI Unit 8 |
|
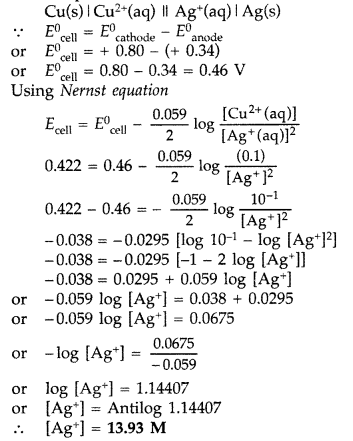
Class -XII Chemistry Chapter-3 Electrochemistry Solved Examples
The standard electrode potential of zinc ions is 0.76V. What will be the potential of a. 2M solution at 300K? Solution: The Nernst equation for the given |
|
Electrochemistr ochemistr ochemistry Electrochemistr ochemistr
65 Electrochemistry. As mentioned earlier (Class XI Unit 8) a galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous. |
|
Chemistry Notes for class 12 Chapter 3 Electrochemistry .pdf
Nernst equation and Kc. At equilibrium. Page 9. 9 |
|
Formulae For CHEMICAL KINETICS
chemical equation. It can be experimentally determined. XII Chemistry. CHAPTER 4 - CHEMICAL KINETICS. If rate law expression for a reaction is. |
|
Test4 ch19 Electrochemistry Practice-answers-Marked
p12. Predictable Oxidation and Reduction Strength Patterns Key Equations Given for Test: ... Determine all oxidation numbers and see which change! |
|
(968)-chemistry-gyan-sutra-jee-main.pdf
12. 6. Ionic Equilibrium. 15. 7. Electrochemistry Formula : Oxidation Number = number of electrons in the valence shell. |
|
Notes - 06 Electrochemistry - CIE Chemistry A Level
6 : Electrochemistry In an ion the sum of the oxidation states of all the atoms is ?equal to the ... Therefore the balanced equation can be written as:. |
Key Information
Electrochemistry is the branch of chemistry that deals with the study of the interchange of chemical and electrical energy. It encompasses a wide range of topics, including redox reactions, electrochemical cells, and the principles of electrochemical measurements.
Examples
Examples of electrochemical processes include the electrolysis of water, the corrosion of metals, and the operation of batteries.
Exercises
Practice 1: Calculate the standard cell potential for the following reaction: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
Solution 1: The standard cell potential can be calculated using the standard reduction potentials of the half-reactions involved.
Practice 2: Determine the equilibrium constant for the following redox reaction: 2CrO4^2-(aq) + 2H3O+(aq) + 3Pb(s) → 2Cr3+(aq) + 3Pb^2+(aq) + 7H2O(l)
Solution 2: The equilibrium constant can be calculated using the Nernst equation and the standard reduction potentials of the half-reactions.
Practice 3: Calculate the cell potential at nonstandard conditions for the following electrochemical cell: Pt(s) | H2(g, 0.50 atm) | H+(aq, 0.10 M) || Cu2+(aq, 0.020 M) | Cu(s)
Solution 3: Use the Nernst equation to calculate the cell potential at nonstandard conditions.
Subcategories
Electrochemistry encompasses multiple subcategories, including redox reactions, electrolysis, electrochemical cells, and electrode potentials.
Notes
1. Electrochemical cells involve the conversion of chemical energy to electrical energy.
2. The standard hydrogen electrode (SHE) is often used as a reference in electrochemistry.
3. Electrolysis is the process of using electrical energy to drive non-spontaneous chemical reactions.
4. The Nernst equation relates the measured cell potential to the concentrations of reactants and products in nonstandard conditions.
Step-by-Step Guide
- Identify the oxidation and reduction half-reactions in the given electrochemical process.
- Balance the chemical equation for the overall reaction and the half-reactions.
- Calculate the standard cell potential using the standard reduction potentials of the half-reactions.
- For nonstandard conditions, use the Nernst equation to calculate the cell potential.
Cases and Scenarios
Case 1: Determining the products of electrolysis of molten sodium chloride.
Case 2: Calculating the cell potential for a galvanic cell based on given half-reactions.
Case 3: Analyzing the corrosion of iron and its prevention using electrochemical methods.
Questions & Answers
Q: What is the relationship between the standard cell potential and the equilibrium constant?
A: The standard cell potential is directly related to the equilibrium constant through the equation ΔG° = -nFE°, where ΔG° is the standard Gibbs free energy change, n is the number of moles of electrons transferred, F is the Faraday constant, and E° is the standard cell potential.
Q: How does the concentration of reactants and products affect the cell potential under nonstandard conditions?
A: According to the Nernst equation, the cell potential is logarithmically related to the concentrations of reactants and products, such that an increase in product concentration or a decrease in reactant concentration leads to a higher cell potential.
Q:
|
Formulae For ELECTROCHEMISTRY - WordPresscom
Unit of Λm in above formula is Scm2mol-1 13 α c m 0 m ∧ = ∧ 14 2 a cα K = 1-α XII Chemistry CHAPTER 3 - ELECTROCHEMISTRY u Important Terms |
|
7 ELECTROCHEMISTRY - Mahesh Tutorials Science
Write the chemical equations for all the steps involved in the rusting of iron, Give any one method to prevent rusting of iron Sol Anode: 2 Fe s Fe aq 2e , + |
|
Electrochemistry all formulas pdf - f-static
Important notes chemistry electrochemistry pdf download are available here for free Comments comments Here are all formulas for electrochemistry class 12 |
|
Electrochemistry - NCERT
As mentioned earlier (Class XI, Unit 8) a galvanic cell is an Equation We have assumed in the previous section that the concentration of all the species Equation Example 3 1 Represent the cell in which the following reaction takes place |
|
CHEMISTRY CLASS-XII - edudel
Analysis shows that a metal oxide has the empirical formula M 0 98 O 1 00 (b ) Atoms B occupy all the octahedral voids and half of the tetrahedral voids Electrochemistry may be defined as the branch of chemistry which deals with the |
|
Unit 3 Electrochemistry - PUE
Electrochemistry One mark Write Nernst Equation ⎡ ⎤ ⎣ ⎦ S H E is assigned an electrode potential of 0 0 V at all temperatures 3 Explain the |
|
Chemistry Class Xii Formulae - Glenn Howells Architects
CBSE CLASS 12 CHEMISTRY IMPORTANT FORMULAS ALL CHAPTERS ' Important Formulae XII Physical Chemistry Electrochemistry May 6th, 2018 |
|
Class -XII Chemistry Chapter-3 Electrochemistry Solved Examples
Class -XII Chemistry Chapter-3 Electrochemistry Solved Examples on The Nernst equation for the given conditions can be written as follows; Molar conductivity of a solution at a dilution V is the conductance of all the ions produced |
|
1310 Electrochemistry Name Symbol Definition SI unit - iupac
(3) v+ and v- are the numbers of cations and anions per formula unit of an difference for zero current through the cell, all local charage transfer equilibria and |
|
Electrochemistry
By 1812, the Swedish chemist Berzelius could propose that all atoms are cell reaction proceeds to the right according to the net equation are shown on the right very similar to that of an ordinary hydrogen electrode, but of course without |