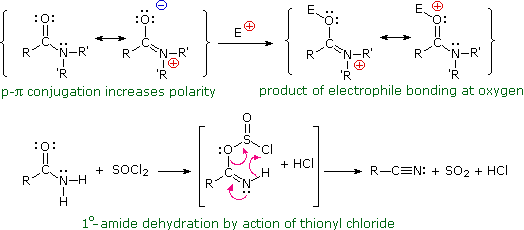
amide to carboxylic acid reaction
How do amines convert carboxylic acids to amides?
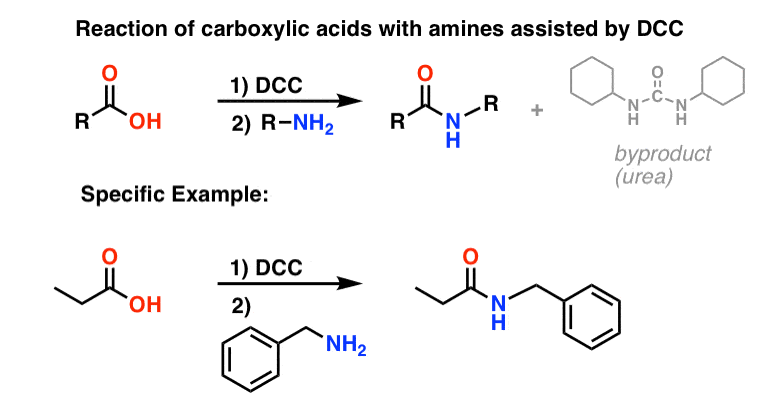
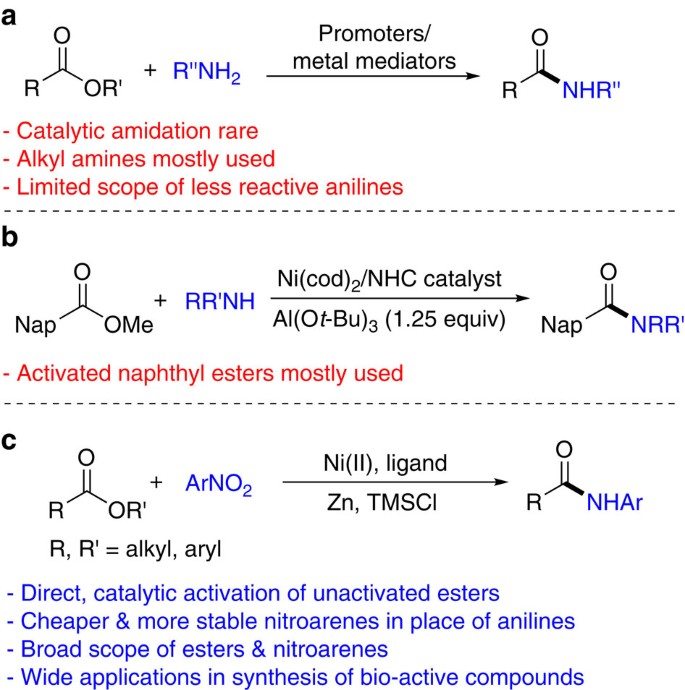
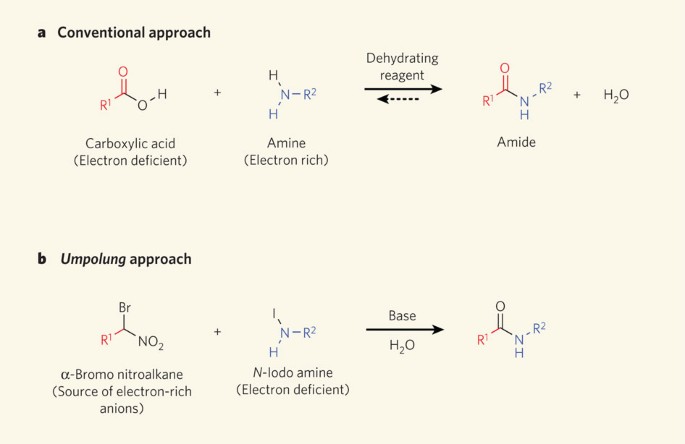
The direct conversion of a carboxylic acid to an amide is difficult because amines are basic and tend to convert carboxylic acids to their highly unreactive carboxylates. In this reaction the carboxylic acid adds to the DCC molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution.
What happens when a carboxylic acid is added to a molecule?
In this reaction the carboxylic acid adds to the DCC molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. DCC induced coupling to form an amide linkage is an important reaction in the synthesis of peptides.
What happens when a carboxylic acid reacts with ammonia (NH3)?
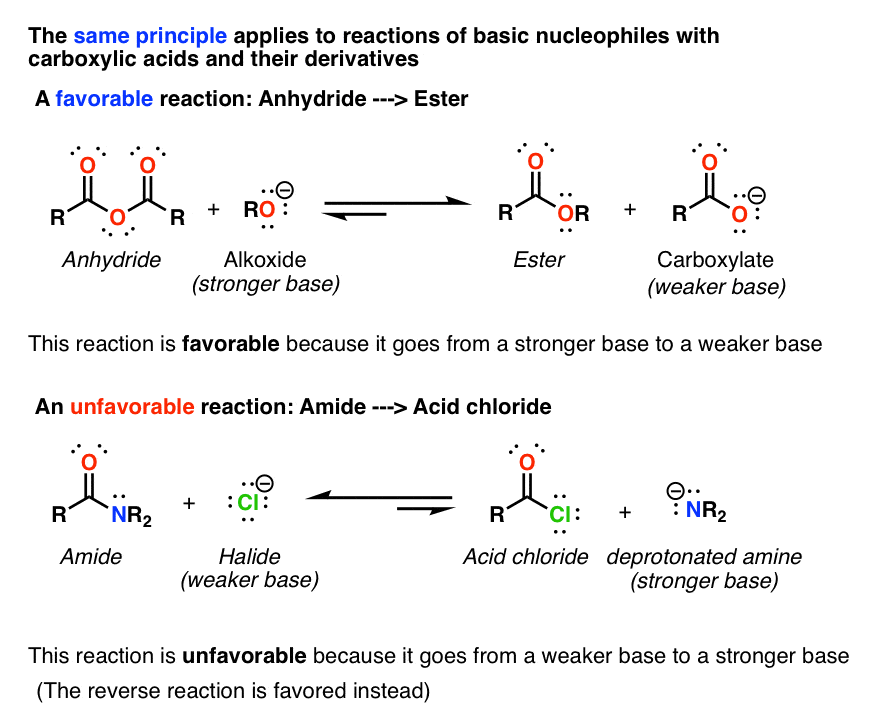
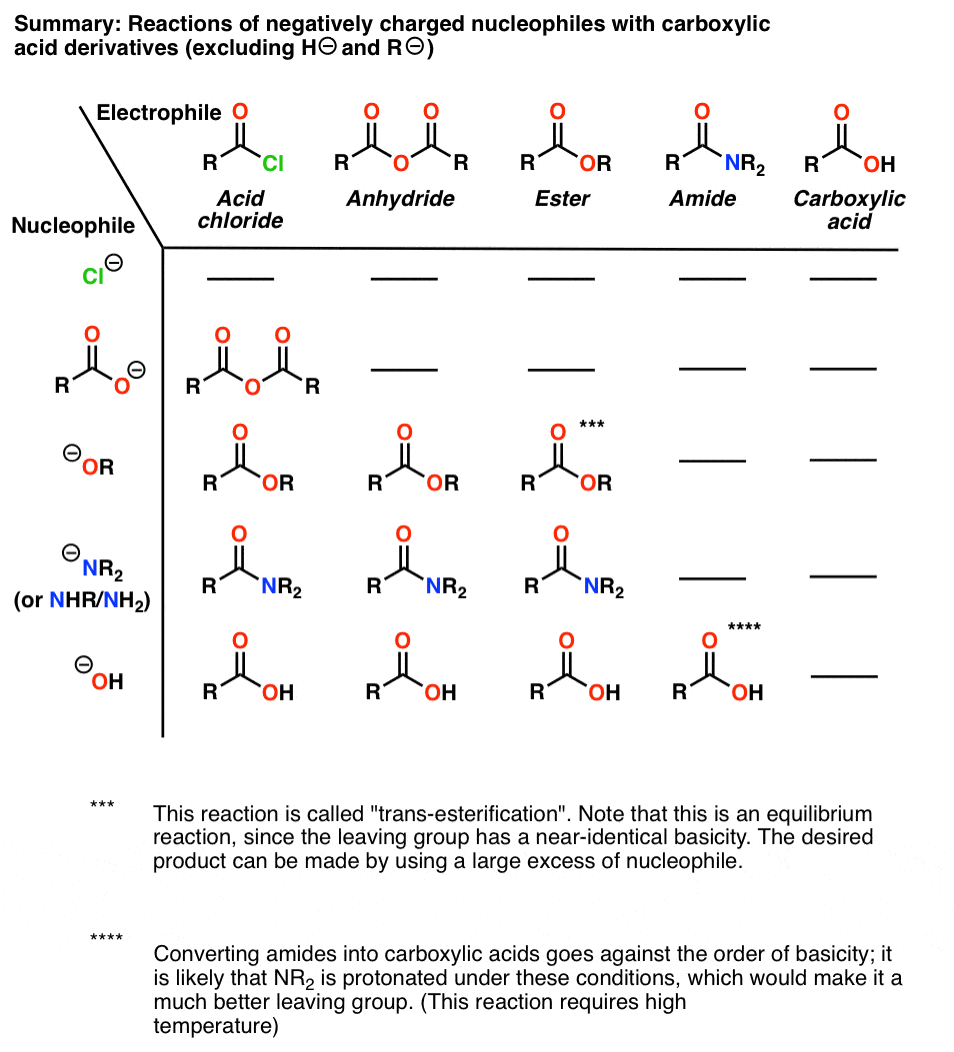
When a carboxylic acid reacts with ammonia (NH 3) a primary amide is formed: When a carboxylic acid reacts with primary or secondary amines, secondary or tertiary amides are produced, respectively. Tertiary amines do not have a hydrogen attached to the nitrogen and therefore do not form amides when mixed with carboxylic acids.
Why is DCC used to convert carboxylic acid to amide?
The direct conversion of a carboxylic acid to an amide is difficult because amines are very basic and tend to convert carboxylic acids to their highly unreactive carboxylate ions. Therefore, DCC (Dicyclohexylcarbodiimide) is used to drive this reaction.
Hydrolysis of Amides
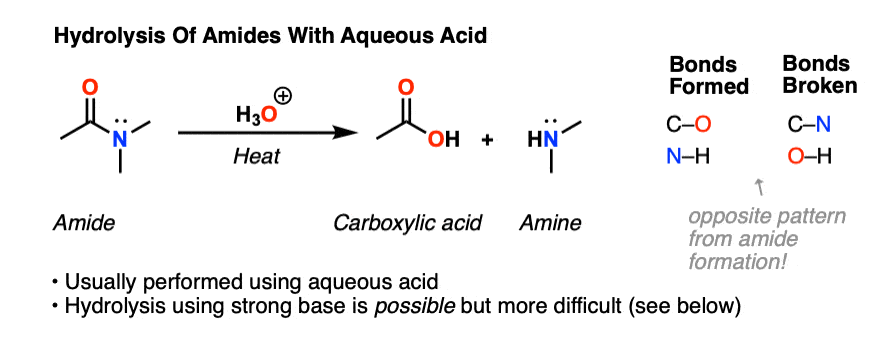
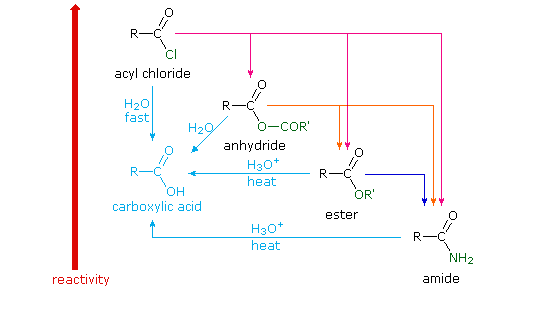
Amides are carboxylic acid derivatives where the –OH of the carboxylic acid has been replaced by –NH2, –NHR, or –NR2 of an amine. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water, this is an example of a “condensation reaction”. [We discuss the nomenclature and synthesis of amides here]. When two amino
Hydrolysis of Amides Using Aqueous Acid: Mechanism
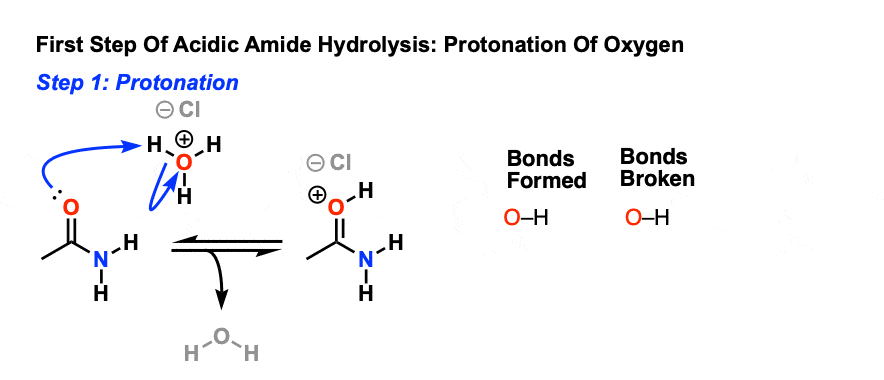
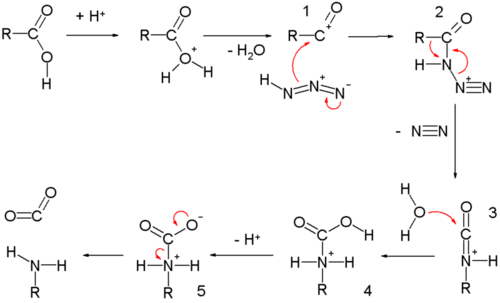
All this is to say that performing the hydrolysis of an amide is not nearly so easy as cleaving an acid halide. The mechanism is also not as simple. So how does the reaction work? As we noted, the first step is the reversible protonation of the amide on oxygen to give the conjugate acid. Protonation of the carbonyl oxygen makes the carbonyl carbon
What About Basic Hydrolysis of Amides?
So that’s acidic hydrolysis. What about basic hydrolysis? It can be done, but it’s typically not easy. If brute force is insisted upon, it’s possible. Hydrolysis of amides with base requires prolonged heating. The whole problem is that in order for a substitution reaction to occur (whether it be SN2 or acyl substitution) you need a decent leaving g
Summary: Hydrolyzing Amides to Carboxylic Acids with Acid Or Base
Acidic hydrolysis of amides is one of those “meat and potatoes” reactions of chemistry that are essential to know and understand. One key to thoroughly understanding the mechanism is to break the reaction down to its six steps (PADPED) and compare it to reactions that share this core mechanistic pathway (e.g. the Fischer Esterification, hydrolysis
Notes
Note 1. Some studies suggest that breakage of the C-N bond does not occur until the secondOH group is deprotonated. masterorganicchemistry.com
Supplemental: 3 Amides That Are Unusually Easy to Break
Amides That Are Unusually Easy To Break (1) – Acylimidazole As we said, amides tend to be difficult to cleave. However it’s worth looking at some exceptions that help to illustrate the key points here. One particularly easy amide to break is acyl imidazole. There’s still a C-N bond, and there’s still a lone pair on nitrogen. So why is it so easy to

Amide formation from carboxylic acid derivatives. Chemistry Khan Academy

Preparation of amides using DCC Organic chemistry Khan Academy

How to Make Amides: Mechanism
|
Part I: Direct Synthesis of Amides from Carboxylic Acid and Amine
Amide bonds are generally prepared by the coupling of carboxylic acids and amines using catalyst. In this thesis we have been studied the amidation reaction of |
|
Hydrolysis of Amides to Carboxylic Acids Catalyzed by Nb2O5
25 ?.?. 2563 KEYWORDS: Nb2O5 Catalyst Amide hydrolysis |
|
Formation of amides: one-pot condensation of carboxylic acids and
The reaction proceeds with low yields when both the carboxylic acid and the amine are sterically hindered. The process takes place. |
|
Www.rsc.org/advances
Carboxylic amides are typically obtained from amines and activated carboxylic acid derivatives through a nucleophilic acyl substitution reaction. |
|
Carboxylic acid participation in amide hydrolysis. External general
A scheme to account for the observed patterns of reaction in carboxylic acid promoted amide hydrolysis is presented which involves intermediates of |
|
Direct Synthesis of Amides from Carboxylic Acids and Amines Using
16 ??.?. 2556 The reactions also typically require relatively dilute reaction conditions. Stoichiometric boron reagents for amidation often require anhydrous ... |
|
Direct Amide Formation Between Carboxylic Acids and Amines
The most desirable solution to this problem of amide formation is the direct condensation i.e. by direct reaction between an amine and a carboxylic acid. |
|
Short Report
15 ?.?. 2559 Appel Reaction of Carboxylic Acids with Tribromoisocyanuric Acid/ ... Esters and amides can be prepared by reacting a carboxylic acid (1 ... |
|
Chapter 6 Amines and Amides
Amides are named by changing the -oic acid ending of the corresponding carboxylic acid to -amide. If alkyl groups are attached to the nitrogen they are named |
|
Hydrolysis of amides to carboxylic acids using phthalic anhydride
Amides efficiently and rapidly give carboxylic acids in high yields upon reaction with phthalic anhydride under microwave irradiation in the absence of |
|
Chapter 9 Lecture Notes: Carboxylic Acids, Amines, and Amides
With heat and an acid catalyst, an amide can be hydrolyzed to produce a carboxylic acid and an amine (or ammonia) A specific example of this reaction is the hydrolysis of N-methylpropanamide |
|
Direct amide formation from unactivated carboxylic acids and
Where the reaction had gone to 100 conversion, the reaction mixture was passed through a short plug of silica to remove the catalyst; otherwise the amides were |
|
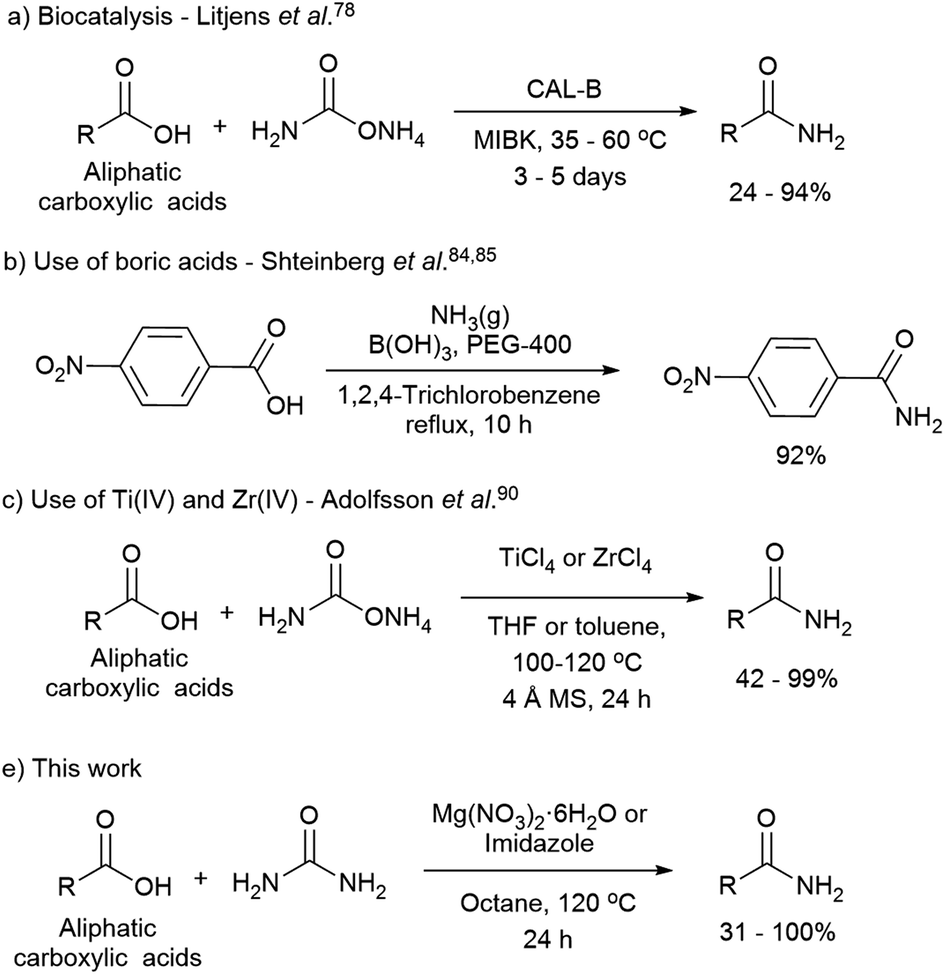
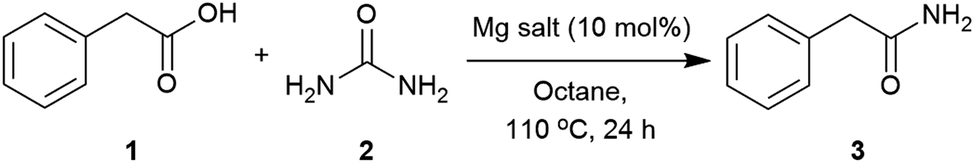
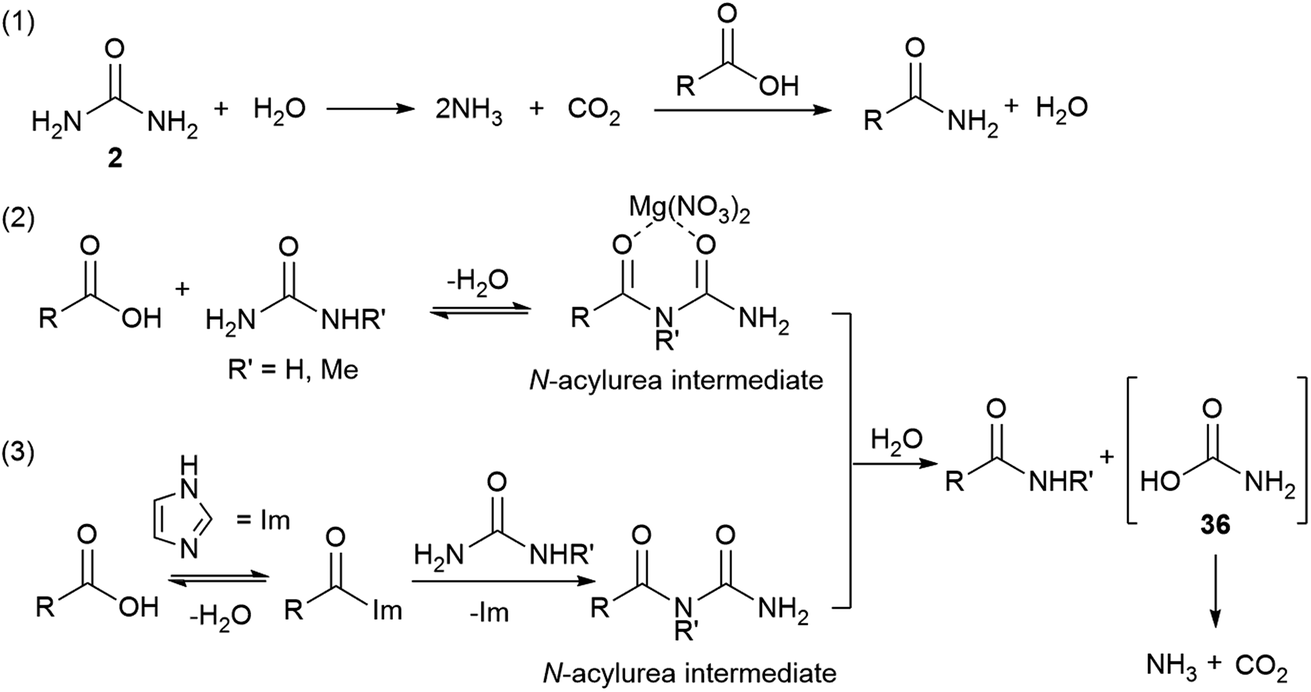
Direct preparation of primary amides from carboxylic acids and urea
primary amides by direct reaction of carboxylic acid and urea in the presence of imidazole under microwave irradiation (Scheme 1) Imidazole was used in this |
|
Chapter 13 Carboxylic Acids, Esters, Amines, and Amides
is the reaction of an ester with a strong base • produces the salt of the carboxylic acid and an alcohol O CH |
|
Carboxylic Acids A carbonyl with one OH attached is called a
Esters can react to form either amides or carboxylates Follows same nucleophilic reaction pathway as seen with other carboxylic acid derivatives O O O O O |