amine and carboxylic acid reaction mechanism
How does a carboxylic acid react with an amine?
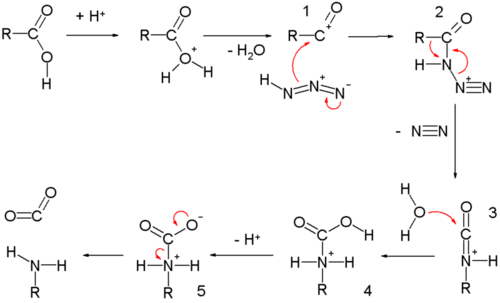
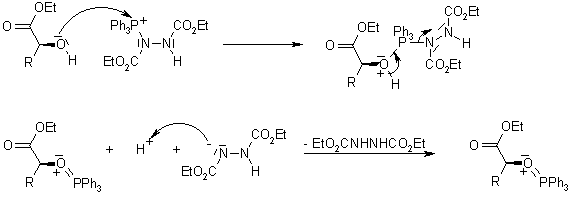
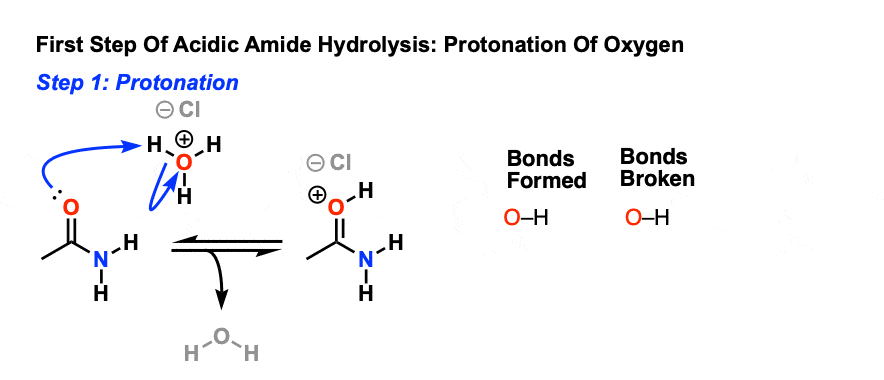
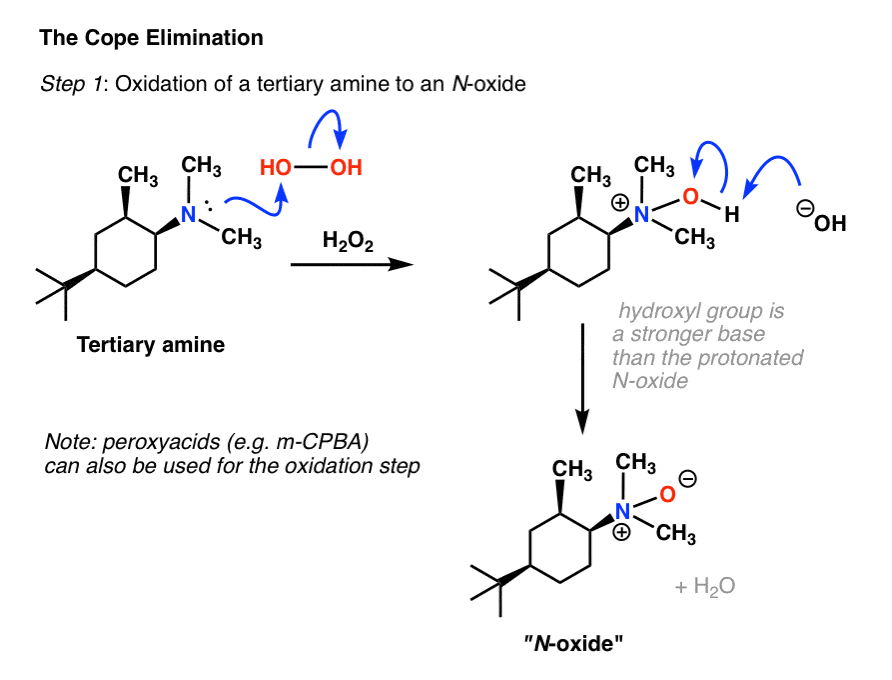
A carboxylic acid first adds to the DCC molecule to form a good leaving group, which can then be displaced by an amine during nucleophilic substitution to form the corresponding amide. The reaction steps are shown below: Step 1: Deprotonation of the acid. Step 2: Nucleophilic attack by the carboxylate. Step 3: Nucleophilic attack by the amine.
How are amides and carboxylic acids United?
Amines and carboxylic acids are two widely available functional groups that are classically united through the amide coupling reaction ( cf. 1 + 2 → 3, Fig. 1a ), a tried-and-true chemistry that has become the most popular reaction for pharmaceutical explorations of chemical space 1.
Overview
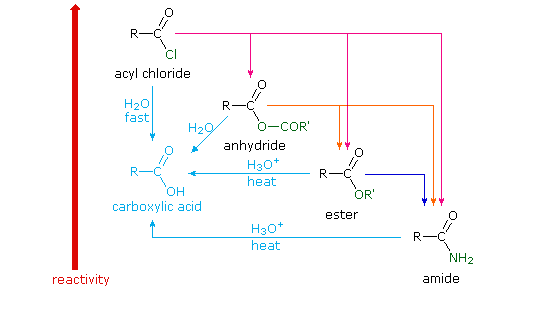
Carboxylic acids belong to a class of organic compounds in which a carbon (C) atom is bonded to an oxygen (O) atom by a double bond and to a hydroxyl group (−OH) by a single bond. A fourth bond links the carbon atom to a hydrocarbon group (R). The carboxyl (COOH) group is named after the carbonyl group (C=O) and hydroxyl group. In general, carboxylic acids undergo a nucleophilic substitution reaction where the nucleophile (-OH) is substituted by another nucleophile (Nu). The carbonyl group (C=O) gets polarized (i.e. there is a charge separation), since oxygen is more electronegative than carbon and pulls the electron density towards itself. As a result, the carbon atom develops a partial positive charge (δ+) and the oxygen atom develops a partial negative charge (δ-). In some cases, in the vicinity of a strong electrophile, the partially negatively charged carbonyl oxygen (δ-) can act as a nucleophile and attack the electrophile (as you will notice in the example of acid chloride synthesis, discussed later in this tutorial). Compounds in which the −OH group of the carboxylic acid is replaced by other functional groups are called carboxylic acid derivatives, the most important of which are acyl halides, acid anhydrides, esters, and amides. •Each derivative contains a common group, termed as an acyl group (R-C=O), which is attached to a heteroatom •They can all be synthesized from the “parent” carboxylic acid •They are all formed through a nucleophilic substitution reaction khanacademy.org
Overview of the reactions that we would be discussing in this tutorial
Let’s list down some common properties for the above shown carboxylic acid derivatives •Each derivative contains a common group, termed as an acyl group (R-C=O), which is attached to a heteroatom •They can all be synthesized from the “parent” carboxylic acid •They are all formed through a nucleophilic substitution reaction •On hydrolysis (i.e. reaction with H2 O), they all convert back to their parent carboxylic acid Now let’s discuss each carboxylic acid derivative individually, and outline the reaction mechanism by which they are formed starting from the parent carboxylic acid khanacademy.org
Acid chloride (ROCl)
Acid chlorides are formed when carboxylic acids react with thionyl chloride (SOCl2 ), PCl3 or PCl5 . They are the most reactive derivatives of carboxylic acid. khanacademy.org
Mechanism of acid chloride formation with SOCl2
(Please follow the movement of electrons carefully) The electrophilic sulfur atom is attacked by the nucleophilic oxygen of carboxylic acid to give an intermediate six membered transition state; which immediately decomposes to the intermediate (A) and HCl respectively. This intermediate (A) then reacts with the HCl molecule, just produced, to give an intermediate (B) which then collapses to form the corresponding acyl chloride, sulfur dioxide and hydrogen chloride. This final step is irreversible because the byproducts, SO2 and HCl, are gases that evaporate off and thus push the reaction in the forward direction. khanacademy.org
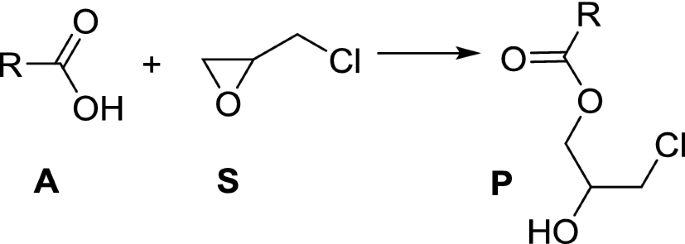
Ester (RCOOR’)
Esters are derived when a carboxylic acid reacts with an alcohol. Esters containing long alkyl chains (R) are main constituents of animal and vegetable fats and oils. Many esters containing small alkyl chains are fruity in smell, and are commonly used in fragrances. The acid-catalyzed esterification of carboxylic acids with alcohols to give esters is termed Fischer esterification khanacademy.org
Thioester (RCOSR’)
Thioesterification: A thioester is formed when a carboxylic acid reacts with a thiol (RSH) in the presence of an acid. Thioesters are commonly found in biochemistry, the best-known example being acetyl CoA. khanacademy.org
Acid anhydride
As you can see, an acid anhydride is a compound that has two acyl groups (R-C=O) bonded to the same oxygen atom. Anhydrides are commonly formed when a carboxylic acid reacts with an acid chloride in the presence of a base. Let’s now discuss the mechanism by which a carboxylic acid anhydride is synthesized. Similar to the Fischer esterification, this reaction follows an addition-elimination mechanism in which the chloride anion (Cl- ) is the leaving group. In the first step, the base abstracts a proton (H+ ) from the carboxylic acid to form the corresponding carboxylate anion (1). The carboxylate anion's negatively charged oxygen attacks the considerably electrophilic acyl chloride's carbonyl carbon. As a result, a tetrahedral intermediate (2) is formed. In the final step, chloride - a good leaving group - is eliminated from the tetrahedral intermediate to yield the acid anhydride. khanacademy.org
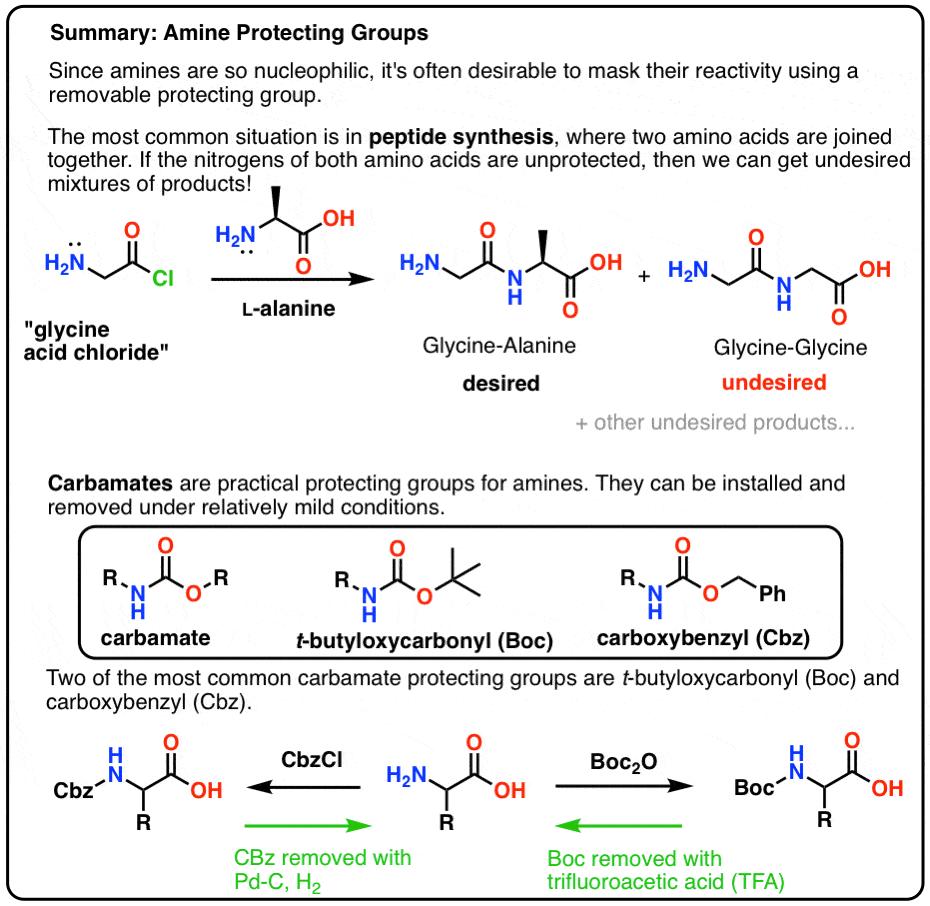
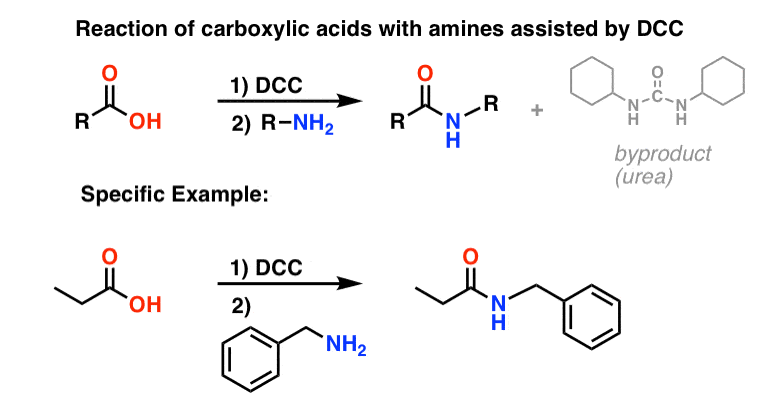
Amide
The direct conversion of a carboxylic acid to an amide is difficult because amines are very basic and tend to convert carboxylic acids to their highly unreactive carboxylate ions. Therefore, DCC (Dicyclohexylcarbodiimide) is used to drive this reaction. The structure of DCC is shown below A carboxylic acid first adds to the DCC molecule to form a good leaving group, which can then be displaced by an amine during nucleophilic substitution to form the corresponding amide. The reaction steps are shown below: Step 1: Deprotonation of the acid. Step 2: Nucleophilic attack by the carboxylate. Step 3: Nucleophilic attack by the amine. khanacademy.org
Relative reactivity of the carboxylic acid derivatives towards a nucleophilic substitution reaction
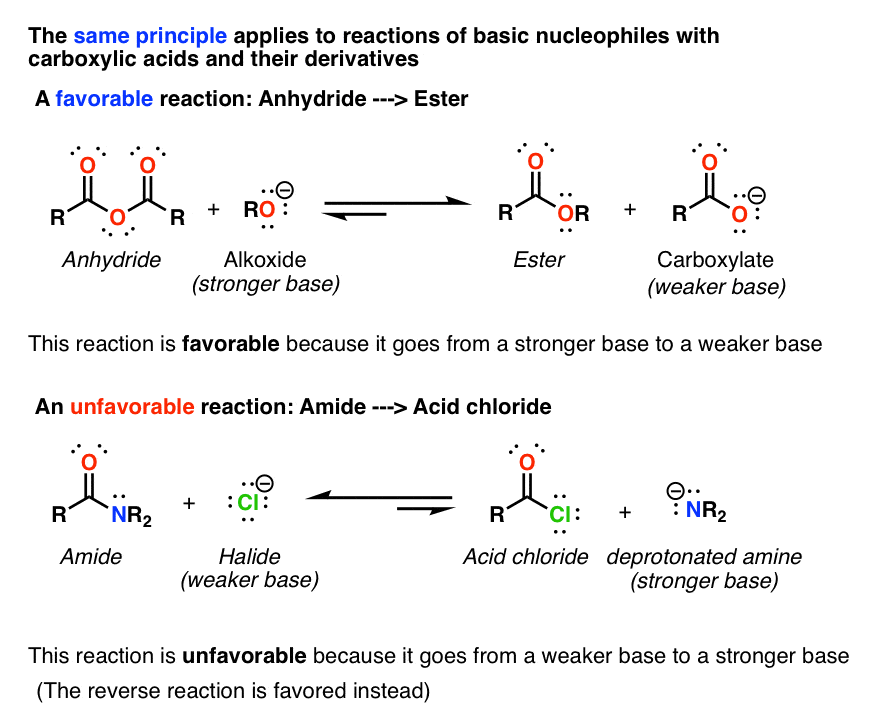
Let’s view the carboxylic acid derivatives as an acyl group, R-C=O, attached to a substituent (X). These derivatives also undergo a nucleophilic substitution reaction with a nucleophile (Nu) as shown above. The reactivity of these derivatives towards nucleophilic substitution is governed by the nature of the substituent X present in the acid derivative •if the substituent (X) is electron donating, it reduces the electrophilic nature of the carbonyl group by neutralizing the partial positive charge developed on the carbonyl carbon, and thus makes the derivative less reactive to nucleophilic substitution •if the substituent (X) is electron withdrawing, then it increases the electrophilic nature of carbonyl group by pulling the electron density of the carbonyl bond towards itself, making the carbonyl carbon more reactive to nucleophilic substitution Thus, on a reactivity scale, the order of reactivity of various carboxylic acid derivatives towards nucleophilic substitution is as follows: Acid halide > acid anhydride > thioester > ester > amide [Attribution and references] khanacademy.org
Want to join the conversation?
Log in khanacademy.org

How to Make Amides: Mechanism

Amine Synthesis Reactions

Carboxylic Acid Derivative Reactions
|
Chapter 5 Carboxylic Acids and Esters
Learn the major chemical reaction of carboxylic acids and esters and learn how to predict the products of ester synthesis and hydrolysis reactions. • Learn |
|
Chapter 6 Amines and Amides
acid ending of the corresponding carboxylic acid to -amide. If alkyl ... Reaction of an amine with acid to produce an alkylammonium salt. amine hydrochloric acid. |
|
Reactions of Amines
Mechanism: Required (protonation). • Reverse Mechanism: Required (deprotonation). • Amines are completely converted to ammonium salts by acids. |
|
The Reaction Mechanism of Polyamide Synthesis by Phosphorylation
competitive reactions of the phosphite-imidazole complex between amine and carboxylic acid which resulted in the molar balance loss of amine or carboxylic acid |
|
Direct Amide Formation Between Carboxylic Acids and Amines
Acceptance of the feasibility and general applicability of this reaction depends upon the development of both an understanding of the mechanism of the reaction |
|
Significance of reagent addition sequence in the amidation of
Feb 19 2015 I2 was investigated in the reaction between benzoic acid and benzyl amine as ... carboxylic acid and the amine moieties to participate in an. |
|
A Novel Method for the Conversion of Carboxylic Acids to NN
Mar 12 2013 N |
|
Quantitative Analysis of the Reaction between Gliadin and Citric
Jul 28 2011 ... reaction temperature on both carboxyl and amine group ... Possible mechanism for the alkali-catalyzed reaction between citric acid and an amine. |
|
Mechanistic insights into boron-catalysed direct amidation reactions
Jan 2 2018 'mechanism' for boron-mediated amidation reactions involving ... Analysis of carboxylic acid/amine/boronic acid reaction systems is a ... |
|
The Schmidt Reaction. I. Conditions and Reaction Mechanism with
vidual carboxylic acid/azide reactions were usually run simultaneously of acetic acid and the amine hydrochloride shown to be of good purity by ... |
|
Direct Catalytic N-Alkylation of Amines with Carboxylic Acids
17 sept. 2014 With respect to the reaction mechanism two main pathways can be proposed for the alkylation of amines with carboxylic acids (Scheme 5): (1) ... |
|
Direct Amide Formation Between Carboxylic Acids and Amines
Acceptance of the feasibility and general applicability of this reaction depends upon the development of both an understanding of the mechanism of the reaction |
|
Accelerated microdroplet synthesis of benzimidazoles by
14 juil. 2020 We provide evidence for an acid catalyzed reaction mechanism based on ... explore C–N coupling of amines using protonated carboxylic acids ... |
|
Direct Synthesis of Amides from Carboxylic Acids and Amines Using
16 avr. 2013 The reactions also typically require relatively dilute reaction conditions. Stoichiometric boron reagents for amidation often require anhydrous ... |
|
Chapter 6 Amines and Amides
products of amide synthesis and hydrolysis reactions. • Learn some of the important properties of of the corresponding carboxylic acid to -amide. If. |
|
Direct Catalytic Reductive N-Alkylation of Amines with Carboxylic
9 juil. 2019 multitude of different functional groups present in the starting carboxylic acids and amines. The reaction is scalable and the. |
|
Mechanistic insights into boron-catalysed direct amidation reactions
2 janv. 2018 'mechanism' for boron-mediated amidation reactions involving ... Analysis of carboxylic acid/amine/boronic acid reaction systems. |
|
Untitled
Synthesis of Amides and Amidines by Reaction of Carboxylic Acids and Amines in the Presence of Polyphophoric Acid Trimethylsilyl. Ester (PPSE). |
|
Formation of amides: one-pot condensation of carboxylic acids and
proceeds with low yields when both the carboxylic acid and the amine are Titanium tetrachloride Carboxylic acids |
|
Chapter 5 Carboxylic Acids and Esters
Learn the major chemical reaction of carboxylic acids and esters and learn how to predict the products of ester synthesis and hydrolysis reactions. |
|
Chapter 9 Lecture Notes: Carboxylic Acids, Amines, and Amides
Given the structure of a carboxylic acid, carboxylate ion, ester, amide, or amine Predict the products for the reactions of carboxylic acids with water, alcohols, |
|
Direct Amide Formation Between Carboxylic Acids and Amines
Amide bonds are typically formed from amines and pre-activated carboxylic acid derivatives such as acid chlorides (prepared from thionyl or oxalyl chloride, for example), anhydrides, or by using the carboxylic acid directly with stoichiometric amounts of coupling reagents such as carbodiimides |
|
75 Reactions of Carboxylic Acids and Amines 1) at low temperature
Notice that this second reaction is analogous to the formation of an ester from an alcohol and carboxylic acid in several ways: 1) A water molecule is “split out” |
|
Reactions of Amines
Amines 3 4b Acylation with Carboxylic Acids to From Amides: (Section 20-12) R Application in synthesis: The amine (an o/p director) is often derived from a |
|
Reactions of Amines
Acylation with Carboxylic Acids to From Amides: (Section 20-12) R N R1 Application in synthesis: The amine (an o/p director) is often derived from a nitro ( a |
|
The Uncatalyzed Direct Amide Formation Reaction Mechanism
23 août 2011 · Keywords: Reaction mechanisms / Carboxylic acids / Amines / Amides / Hydrogen bonds Calorimetric studies of the mixing of a series of |