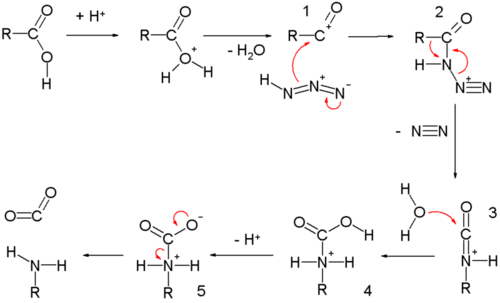
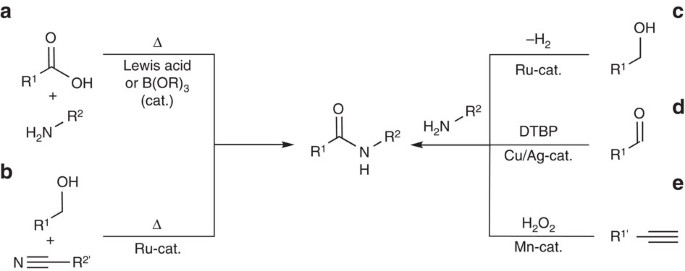
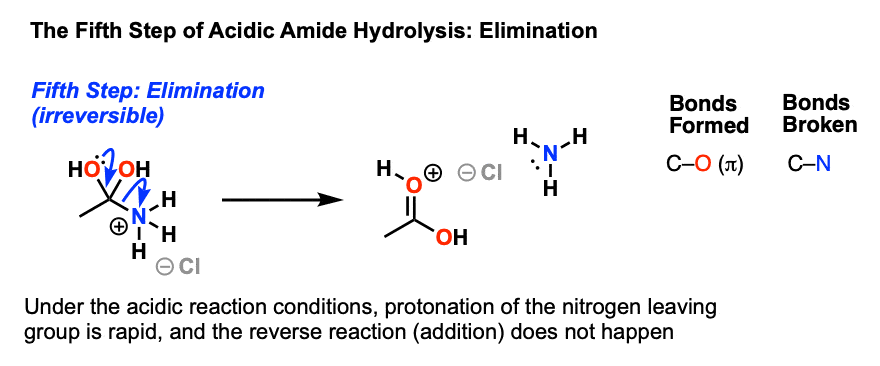
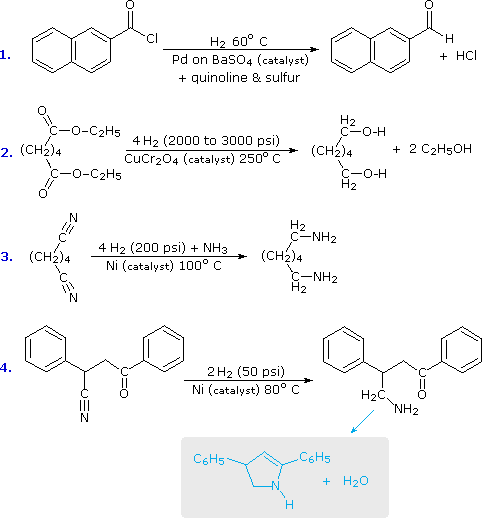
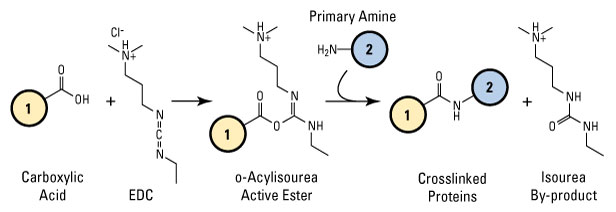
amine carboxylate reaction
Can amides be formed by a carboxylic acid and an amine?
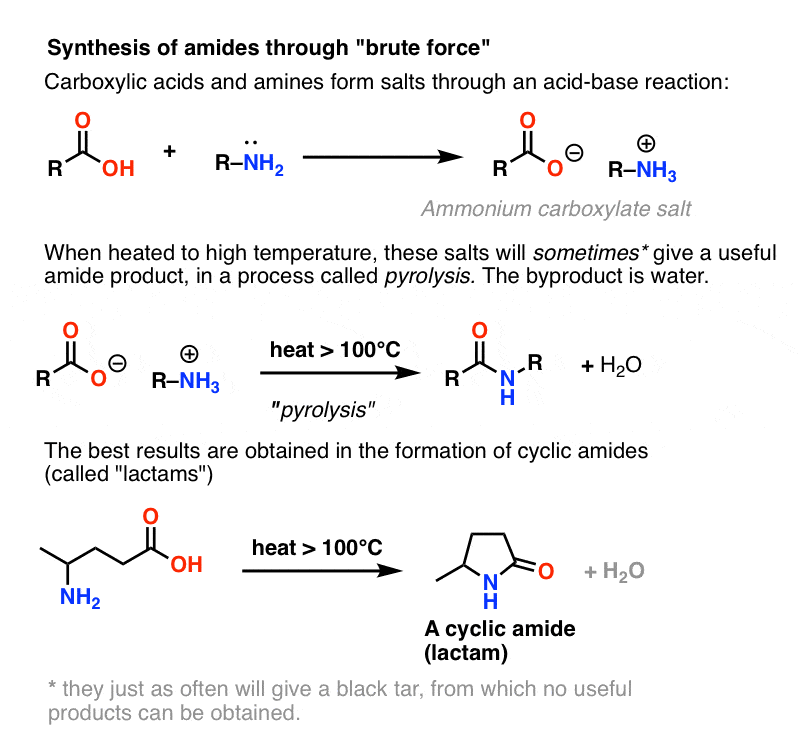
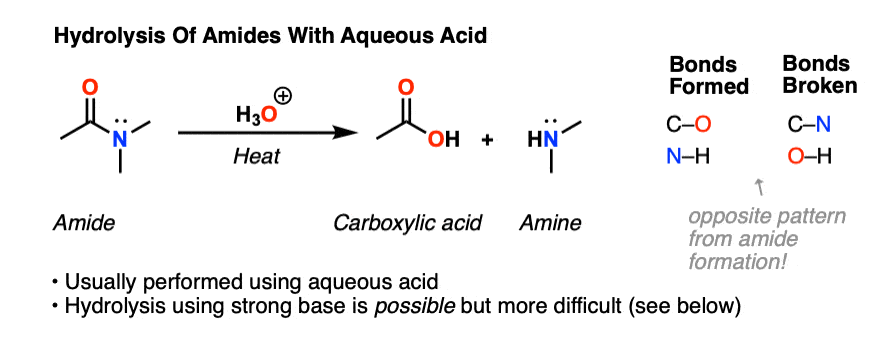
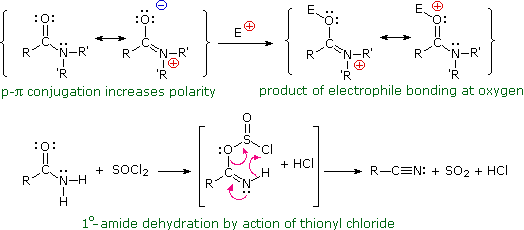
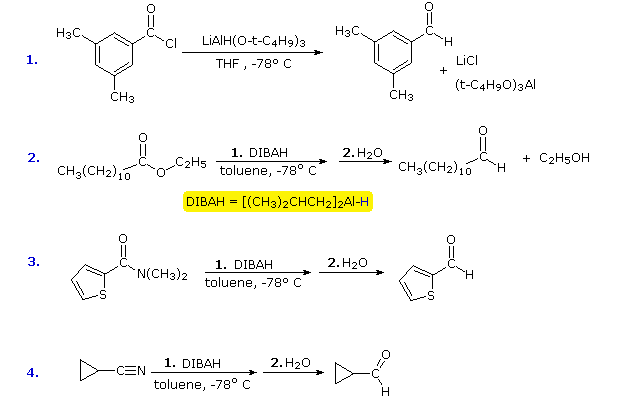
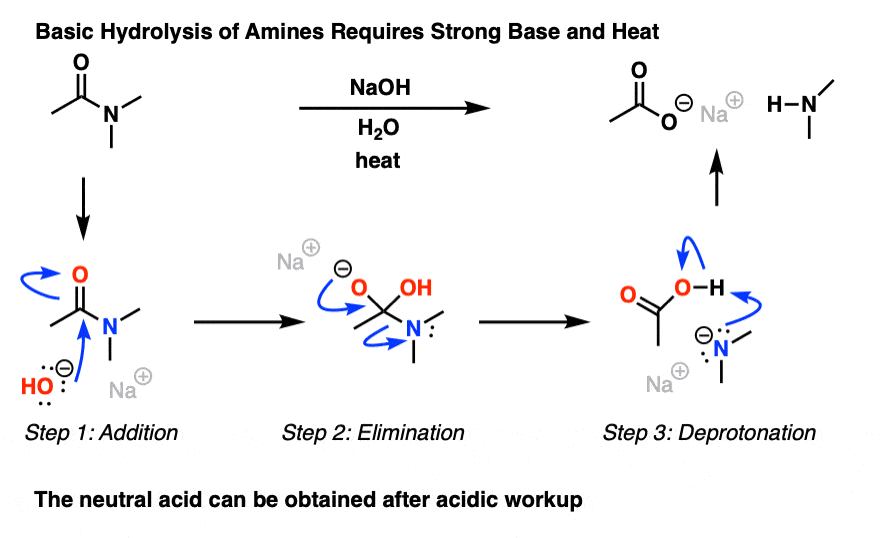
Lastly, amides can be formed through the direct reaction of a carboxylic acid and an amine. However, this reaction is rarely used because the conditions are relatively severe. Amides are relatively unreactive towards nucleophilic acyl substitutions due to the poor leaving group ability of its nitrogen containing Y group.
What happens if amine reacts with carboxylic acid?
The direct reaction of a carboxylic acid with an amine would be expected to be difficult because the basic amine would deprotonate the carboxylic acid to form a highly unreactive carboxylate. However when the ammonium carboxylate salt is heated to a temperature above 100 o C water is driven off and an amide is formed.

Amine Synthesis Reactions

Amine Synthesis Reactions Organic Chemistry

Imine and Enamine Formation Reactions With Reductive Amination
|
INSTRUCTIONS - NHS and Sulfo-NHS
Carboxylates (-COOH) may be reacted to NHS or Sulfo- perform the first reaction in MES buffer (or other non-amine non-carboxylate buffer). |
|
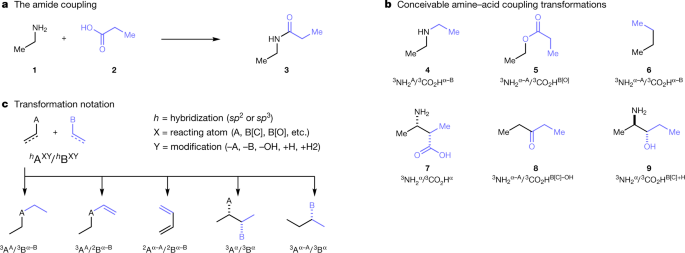
A map of the amine–carboxylic acid coupling system
2 avr. 2020 The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used for drug discovery1. |
|
Formation of amides: one-pot condensation of carboxylic acids and
proceeds with low yields when both the carboxylic acid and the amine are Titanium tetrachloride Carboxylic acids |
|
Coupling of substances containing a primary amine to hyaluronan
The carbodiimide reacts with carboxylic groups of e.g. polysaccharides which is shown briefly in Scheme 1; the reaction between a carboxyl group and |
|
Direct Amide Formation Between Carboxylic Acids and Amines
The most desirable solution to this problem of amide formation is the direct condensation i.e. by direct reaction between an amine and a carboxylic acid. |
|
Preparation and properties of tetrazinc ?4-oxohexa-?-carboxylates
carboxylic acid and zinc oxide was found to be rcstrictcd to branched chain acids. The reaction of these basic salts with primary and sccondary amines Icd |
|
Biotinylate carboxyl groups with EDC and Biotin Hydrazide
EDC reactions are usually performed in acidic buffer (pH 4.7-5.5) but efficient coupling can be accomplished in amine- and carboxylate-. |
|
Rhodium-Catalyzed Asymmetric Ring Opening Reactions of
reactions employing an aromatic amine malonate or carboxylate nucleophile are dramatically improved. Third |
|
Mechanistic insights into boron-catalysed direct amidation reactions
2 janv. 2018 Rapid reaction between amines and boron compounds was observed in all ... Analysis of carboxylic acid/amine/boronic acid reaction systems. |
|
Chapter 6 Amines and Amides
Learn the major chemical reactions of amines and amides and learn how to predict the of the corresponding carboxylic acid to -amide. If. |
|
Chapter 9 Lecture Notes: Carboxylic Acids, Amines, and Amides
Given the structure of a carboxylic acid, carboxylate ion, ester, amide, or amine for the reactions of carboxylic acids with water, alcohols, amines, ammonia, |
|
Carboxylic Acids, Amines, and Amides
carboxylic acid, amine, or amide molecules to one another, and how these forces Predict the products for the reactions of carboxylic acids with water, alcohols |
|
75 Reactions of Carboxylic Acids and Amines 1) at low temperature
Notice that this second reaction is analogous to the formation of an ester from an alcohol and carboxylic acid in several ways: 1) A water molecule is “split out” |
|
Direct amide formation from unactivated carboxylic acids and
crude reaction mixtures were analysed by their 1H NMR and 13C NMR spectra carboxylic acid (1 0 mmol) was added to an oven dried Radleys carousel tube |
|
Reactions of Amines
Reaction with Ketones or Aldehydes (Section 18-16,17 and 19-10) R' R The amine is more basic than the carboxylate, the carboxylic acid more acidic than |
|
Reactions of Amines
Neutral amine can completely deprotonate carboxylic acids, but not water or alcohols 6 Therefore hydroxide can deprotonate ammoniums, but carboxylates |
|
Chapter 13 Carboxylic Acids, Esters, Amines, and Amides
Esterification is the reaction of a carboxylic acid and alcohol in the presence of A tertiary (3°) amine has three carbon groups bonded to the nitrogen atom H |
|
A map of the amine–carboxylic acid coupling system - Nature
2 avr 2020 · The coupling of an amine with a carboxylic acid to form an amide bond is the most popular chemical reaction used for drug discovery1 However, |