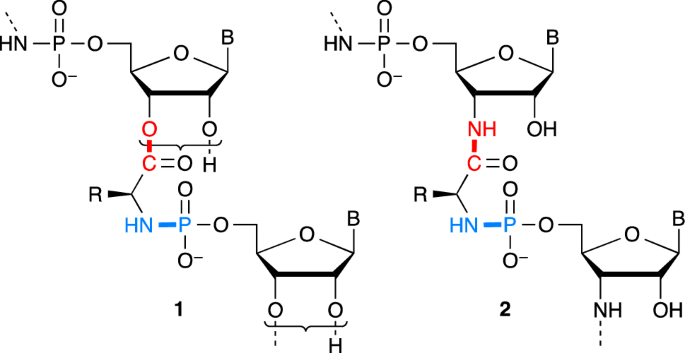
amino amide vs amine ester
Are local anesthetics amine or aromatic?
Most local anesthetics contain an aromatic group and an amine group separated by an intermediate chain (Table 1). The clinically useful local anesthetics fall into one of two chemical groups. Amino-esters (procaine, chloroprocaine and tetracaine) contain an ester link between the aromatic portion and the intermediate chain.
How are esters converted into amides?
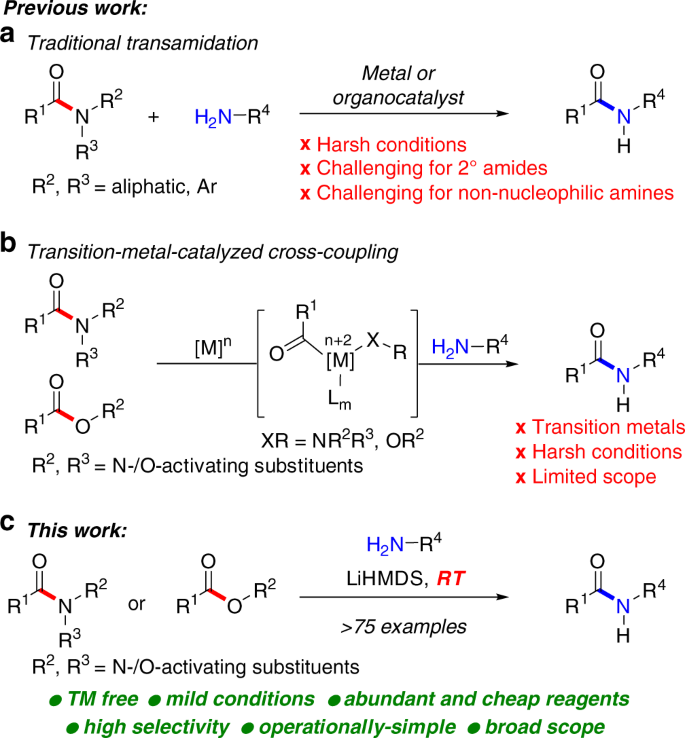
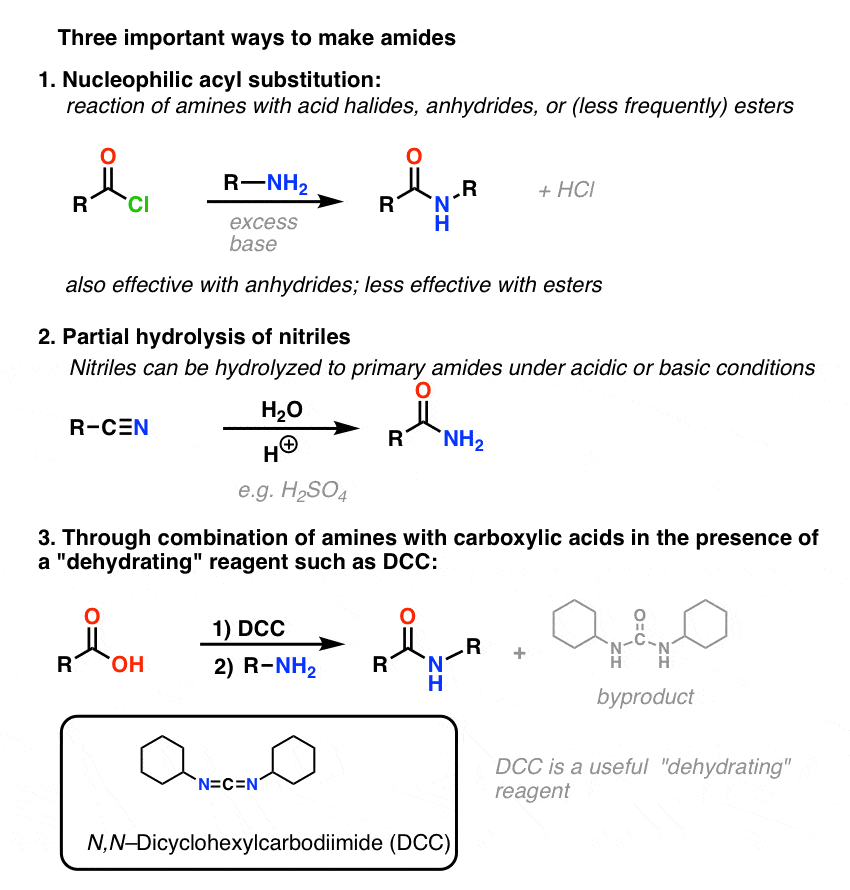
Please let us know in the Reviews section here. Esters can be converted into amides by reacting with amines in an aminolysis reaction. The reaction goes by a nucleophilic addition-elimination mechanism.
Why are amino esters called amide local anaesthetics?
They are named for their ester bond and are unlike amide local anaesthetics. Structurally, amino esters consist of three molecular components: The chemical linkage between the lipophilic part and the intermediate chain can be of the amide -type or the ester-type, and is the general basis for the current classification of local anesthetics.

Ester vs Amide Local Anesthetics How they differ?

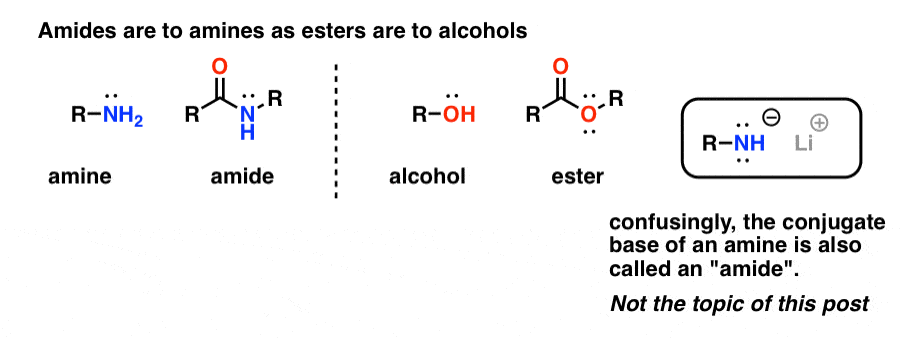
Amines and Amides

Nomenclature and properties of amides Organic chemistry Khan Academy
|
DIFFERENTIAL NERVE BLOCKADE: ESTERS V. AMIDES AND
DIFFERENTIAL NERVE BLOCKADE: ESTERS V. AMIDES aromatic ring is connected to an amine group by ... and with those of a series of amino-ester local. |
|
Chapter 6 Amines and Amides
Learn to recognize the amine and amide functional groups. Nitrogen is in Group V of the periodic table and in ... the amino acid arginine; also. |
|
Degradable Poly(ester amide)s for Biomedical Applications
27 dic 2010 made to get functionalized poly(ester amide)s by incorporation of ?-amino acids with hydroxyl carboxyl and amine pendant groups and also by ... |
|
Amine-Reactive Activated Esters of meso-CarboxyBODIPY
17 apr 2020 Fluorogenic Assays and Labeling of Amines Amino Acids |
|
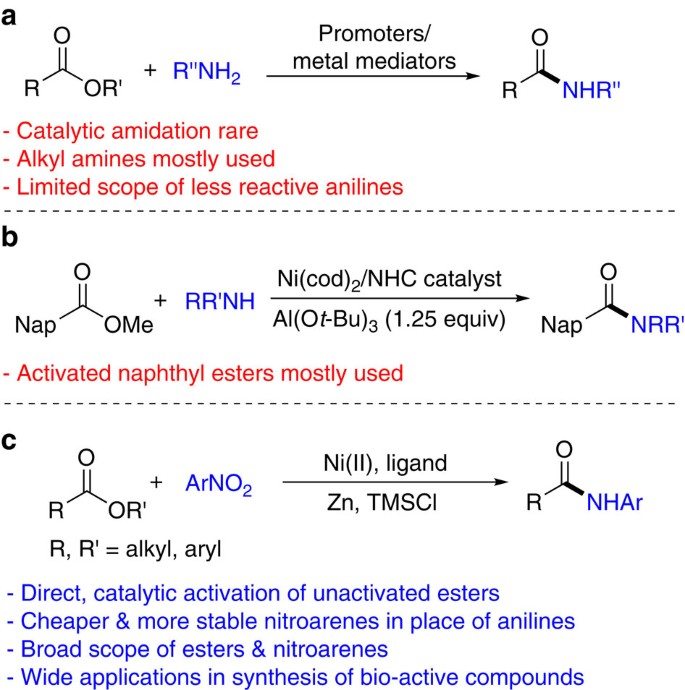
Catalytic Ester?Amide Exchange Using Group (IV) Metal Alkoxide
plated macrolactamization of tetra-amino esters using stoichio- entries 8 (aromatic amine vs aliphatic alcohol) 9 and 10 (ester. |
|
Ester or Amine Which is Better?
4-D%20Amine%20or%20Ester%202004-Purdue.pdf |
|
The aminolysis of N-hydroxysuccinimide esters. A structure-reactivity
ligands.7 Thereport that NHS esters preferentially acylate amino groups under mild reaction conditions8 has other than the amide and the NHS amine salt. |
|
COMMUNICATION Catalytic Ester and Amide to Amine
Catalytic Ester and Amide to Amine Interconversion: Nickel-. Catalyzed Decarbonylative Amination of Esters and Amides via C-. O and C-C Bond Activation. |
|
Functional Group Characteristics and Roles
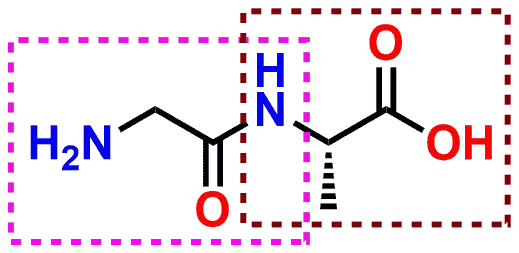
penicillin G and penicillin V is the presence of an ether oxygen atom in As shown in Figure 2-17 each amino acid contains a primary amine |
|
Synthesis of ferrocene amides and esters from aminoferrocene and
17 feb 2017 v = 100 mV s-1. All ferrocene derivatives were systematically oxidized in the presence of a number of amino acids (L-alanine L-. |
|
Anesthésiques locaux
4 fév 2016 · (un ester ou un amide); – Une amine tertiaire Page 14 Structure chimique Dépolarisation cellulaire |
|
Toxicité des anesthésiques locaux - Société Française des Infirmier
groupement amine et une chaîne intermédiaire composée soit d'un groupement du type ester soit Le métabolisme des AL de type amino-amide type amino- esters (procaïne, chloroprocaïne) sont hydrolysés par les pseudocholinestérases |
|
Pharmacologie des anesthésiques locaux
cules ont été synthétisées, d'abord les esters par les chimistes en pratique clinique ont un groupement amine tertiaire situé entre la chaîne tent : les esters et les amides taire, mais leur métabolite, l'acide para-amino-benzoïque, passe |
|
12 Synthèse des esters et des amides - Chimie PCSI
Utiliser la formation des esters et des amides dans le cadre d'une stratégie de Les acides aminés sont des acides carboxyliques porteurs d'un groupe amino |
|
12 Synthèse des esters e
Comment synthétiser, avec un bon rendement, un ester ou un amide à partir Les acides aminés sont des acides carboxyliques porteurs d'un groupe amino |
|
Influence of the structure of amine components on carboxypeptidase
L-Amino acids amides, leucinol hydrochlorid, o-alanine, o-valine, D- alanine amide hydrochloride, N-benzoyl-tyrosine ethyl ester, N-benzoyl-alanine methyl |
|
Anesthésiques locaux – mécanisme daction et risques
sanguin, et les amides par voie enzymatique dans le foie Les esters ne sont plus guère utili- sés car leur métabolite, l'acide para- amino- benzoïque, a un |
|
28 CLASSIFICATION OF LOCAL ANESTHETICS ESTERS AMIDES
ESTERS AMIDES Benzocaine Chloroprocaine* Cocaine Proparacaine Tetracaine* are no longer any injectable ester-type local anesthetic products |
|
Surdosage en anesthsiques locaux - ICARWEB
Les deux familles d'AL à notre disposition (amino-amides et amino-esters) ont pour effet C'est le carbone proximal du groupe amine qui forme le centre |





















![PDF] Synthesis of amides from esters and amines with liberation of PDF] Synthesis of amides from esters and amines with liberation of](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:68560:20160511103824788-0298:72020tbl46_2.gif?pub-status\u003dlive)