p block elements questions and answers pdf
|
I Multiple Choice Questions (Type-I)
137 The p-Block Elements (iii) presence of higher orbitals (iv) higher atomic number 17 The linear shape of CO 2 is due to _____ (i) sp3 hybridisation of carbon (ii) sp hybridisation of carbon (iii) pπ– pπbonding between carbon and oxygen (iv) sp2 hybridisation of carbon 18 Me 3 SiCl is used during polymerisation of organo silicones because |
|
THE p -BLOCK ELEMENTS
(E = element) The nature of these oxides varies down the group Boron trioxide is acidic and reacts with basic (metallic) oxides forming metal borates Aluminium and gallium oxides are amphoteric and those of indium and thallium are basic in their properties |
Why do p block elements have low boiling and melting points?
Also, they can not tolerate heat. Hence, they have a very low boiling as well as melting points as compared to other elements of the same group. Click to download Important NEET Chemistry Questions for P Block Elements in a free PDF file.
How many p block elements questions are there in JEE Main?
You can find a minimum of 4 questions from the P Block Elements chapter in the JEE Main Exam. Here is the table where you can find the weightage of the P Block Elements chapter in the past 5 years of the JEE Main Exam. Practice Papers for JEE Main help you to find and practice the questions that might get asked in the next JEE Main exam.
What are the topics of p block elements?
Here are some of the important topics of P Block Elements. Properties and uses of Boron and Aluminum. Formation and properties of boron hydrides (boranes). Allotropes of carbon (diamond, graphite, graphene). Silicones and their uses. Carbon compounds like carbonates, carbides, and silicates. Properties of nitrogen and phosphorus.
How Vedantu p block elements JEE Main practice paper works?
Vedantu’s P Block Elements JEE Main Practice Paper is composed of MCQs and Subjective type questions. At the end of the FREE PDF you can get the answer keys and detailed solutions for the questions. If you follow the below instructions while working out the Daily Practice Paper you can easily succeed in the JEE Main exam.
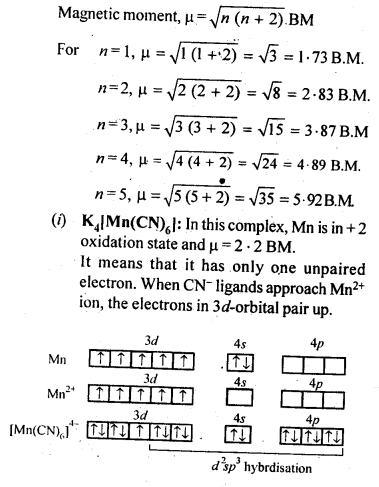
2EN ( s )
(E = element) The nature of these oxides varies down the group. Boron trioxide is acidic and reacts with basic (metallic) oxides forming metal borates. Aluminium and gallium oxides are amphoteric and those of indium and thallium are basic in their properties. ncert.nic.in
Problem 11.2
White fumes appear around the bottle of anhydrous aluminium chloride. Give reason. ncert.nic.in
Solution
Anhydrous aluminium chloride is partially hydrolysed with atmospheric moisture to liberate HCl gas. Moist HCl appears white in colour. ncert.nic.in
11.3 SOME IMPORTANT COMPOUNDS OF BORON
Some useful compounds of boron are borax, orthoboric acid and diborane. We will briefly study their chemistry. ncert.nic.in
Solution
Because it is not able to release H+ ions on its own. It receives OH–ions from water molecule to complete its octet and in turn releases H+ ions. ncert.nic.in
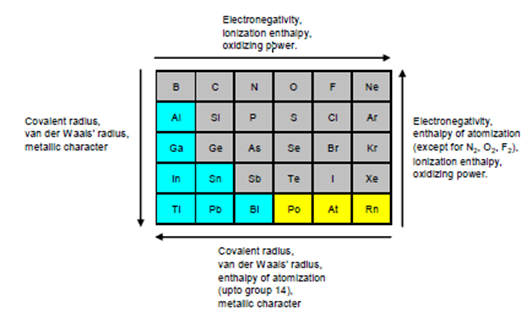
11.5.2 Covalent Radius
There is a considerable increase in covalent radius from C to Si, thereafter from Si to Pb a small increase in radius is observed. This is due to the presence of completely filled d and f orbitals in heavier members. ncert.nic.in
11.6 IMPORTANT TRENDS AND ANOMALOUS BEHAVIOUR OF CARBON
Like first member of other groups, carbon also differs from rest of the members of its group. It is due to its smaller size, higher electronegativity, higher ionisation enthalpy and unavailability of d orbitals. In carbon, only s and p orbitals are available for bonding and, therefore, it can accommodate only four pairs of electrons around it. This
Problem 11.7
Diamond is covalent, yet it has high melting point. Why ? ncert.nic.in
Solution
Diamond has a three-dimensional network involving strong C—C bonds, which are very difficult to break and, in turn has high melting point. ncert.nic.in
11.8.1 Carbon Monoxide
Direct oxidation of C in limited supply of oxygen or air yields carbon monoxide. ncert.nic.in
11.8.2 Carbon Dioxide
It is prepared by complete combustion of carbon and carbon containing fuels in excess of air. ncert.nic.in
Solution
Simple silicones consist of chains in which alkyl or phenyl groups occupy the remaining bonding positions on each silicon. They are hydrophobic (water repellant) in nature. ncert.nic.in
11.8.6 Zeolites
If aluminium atoms replace few silicon atoms in three-dimensional network of silicon dioxide, overall structure known as aluminosilicate, acquires a negative charge. Cations such as Na+, K+ or Ca2+ balance the negative charge. Examples are feldspar and zeolites. Zeolites are widely used as a catalyst in petrochemical republshed industries for crack
|
Class XII Chapter 7 – The p Block Elements Chemistry Page 1 of 27
Question 7.14: Why does nitrogen show catenation properties less than phosphorus? Answer. Catenation is much more common in phosphorous compounds than in |
|
Keep511.pdf
16-Apr-2018 135 The p-Block Elements. 16-04-2018 ... In the following questions two or more options may be correct. ... Answer on the basis of Fig.11.1. |
|
ASPIRATIONS Institute
The first element in p-block element has four valence orbitals i.e. one 2s and three 2p. Following questions can be answered :. |
|
The p - block elements
Diversity in chemistry is the hallmark of p–block elements manifested Solution. Intext Question. 7.3 Why is N2 less reactive at room temperature? |
|
The p-Block Element
23-Apr-2018 Note : In the following questions two or more options may be correct. 28. If chlorine gas is passed through hot NaOH solution two changes are ... |
|
REASONING BASED QUESTIONS FROM P-BLOCK ELEMENTS
REASONING BASED QUESTIONS FROM P-BLOCK ELEMENTS. GROUP-15. 1. Nitrogen does not form pentahalide although it exhibit +5 oxidation state. |
|
B. Sc. II YEAR INORGANIC CHEMISTRY-II
difference from those of s or p-block elements which are white or light compounds paramagnetism is common though some metals in the elemental form. |
|
CBSE NCERT Solutions for Class 12 Chemistry Chapter 7
Practice more on The p-Block Elements Back of Chapter Questions ... What happens when white phosphorus is heated with concentrated NaOH solution. |
|
And f- Block Elements I. Answer the following questions. Each
Ans. The d block elements are in the middle of s and p blocks 3d series transition metals exhibit +2 as the most common oxidation state (except. |
|
REASONING QUESTIONS IN P BLOCK ELEMENTS
What happens when white phosphorus is heated with concentrated NaOH? Solution in an inert atmosphere of CO2 ? Page 2. 15. Why does PCl3 fume in |
What is the easiest way to study p-block elements?
. There are different types of silicates and remembering them is important.
What are the 35 p-block elements?
What elements are p-block elements?
. These include metals, metalloids, noble gases and halogens.
. Some of the commonly known elements in the P-block are: Metals: Aluminium (Al), Boron (B), Tin (Sn).
|
Chapter 10 S -BLOCK ELEMENTS Question and answers carrying 1
Question and answers carrying 2 mark 1 Write the general electronic configuration of s-block elements [noble gas]ns 1 for alkali metals |
|
QUESTION BANK CHAPTER 1 d- BLOCK ELEMENTS
6) Write the outer electronic configuration of chromium (Atomic number; Cr = 24) Q2) Answer the following 2/3 Marks each 1) What are transition element? |
|
Question Bank of d & f block - chemistryworkshopjr
Answers:- Very short answer questions:- Q 1 To aquire nearest noble gas configuration Q 2 In transition elements electron enter into penultimate shell and in |
|
THE s -BLOCK ELEMENTS - NCERT
which makes Li, the strongest reducing agent in aqueous solution (i) Sublimation In the following questions two or more options may be correct 22 Metallic |
|
THE p -BLOCK ELEMENTS - NCERT
16 avr 2018 · The element which exists in liquid state for a wide range of temperature and In the following questions two or more options may be correct 16 hydrochloric acid or dilute sodium hydroxide solution in a test tube and on |
|
5 d & f BLOCK ELEMENTS - Mahesh Tutorials Science
d AND f BLOCK ELEMENTS In solution, the stability of the compounds depends upon EXERCISE - 2 : PREVIOUS YEAR COMPETITION QUESTIONS 2013 |
|
HL Answers to First row d-‐block elements questions - WordPresscom
http://www thinkib net/chemistry HL Answers to First row d-‐block elements questions 1 A transition element contains an incomplete d sub-‐level in one or |
|
The p Block Elements Chemistry Page 1 of 27 Question - askIITians
Why does NH3 form hydrogen bond but PH3 does not? Answer Nitrogen is highly electronegative as compared to phosphorus This causes a greater attraction of |
|
D and f-Block Elements - Target Publications
Explain the position of d-block elements in the periodic table Ans: Position of d- block orbitals in their elementary state or in any of their common oxidation states ii The 3d, 4d, 5d Answers to Multiple Choice Questions 1 (C) 2 (C) 3 ( A) 4 |