analytical concentration definition
|
Chapter 4: Calculations Used in Analytical Chemistry
Mass is an invariant measure of the quantity of matter in an object Weight is the force of attraction between an object and its surroundings principally the earth Because gravitational attraction varies with geographical location the weight of an object depend on where you weigh it For example a crucible weighs less in Denver than in Atlantic |
What is analytical chemistry?
Analytical chemistry consists of classical, wet chemical methods and modern, instrumental methods. Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity.
What does concentration mean in chemistry?
According to IUPAC, the term concentration includes four quantities characterizing the composition of a mixture with respect to the volume of the mixture : mass, amount, volume and number concentration. Also, the term concentration is a shortcut for amount-of-substance concentration or amount concentration.
What is a general method for analysis of concentration?
A general method for analysis of concentration involves the creation of a calibration curve. This allows for the determination of the amount of a chemical in a material by comparing the results of an unknown sample to those of a series of known standards.
4A-2 The Distinction Between Mass and Weight
Mass is an invariant measure of the quantity of matter in an object. Weight is the force of attraction between an object and its surroundings, principally the earth. Because gravitational attraction varies with geographical location, the weight of an object depend on where you weigh it. For example, a crucible weighs less in Denver than in Atlantic
w mg
w is the weight of an object, m is its mass, and g is the acceleration due to gravity. Analytical data are based on mass rather than weight. A balance is used to compare the mass of an object with the mass of one or more standard masses. g affects both unknown and known equally, hence, the mass of the object is identical to the standard masses with
V c
concd concd V dil c dil The two terms on the left are the volume and molar concentration of a concentrated solution that is being used to prepare a diluted solution having the volume and concentration given by the corresponding terms on the right. This equation is based on the fact that the number of moles of solute in the diluted solution must
4C Chemical stoichiometry
Stoichiometry is the quantitative relationship among the amounts of reacting chemical species. The stoichiometry of a reaction is the relationship among the number of moles of reactants and products as represented by a balanced chemical equation. web.iyte.edu.tr
|
Chapter 4: Calculations Used in Analytical Chemistry
Molar concentration molar analytical concentration |
|
Definition of Minimum Performance Requirements for Analytical
20 oct. 2015 Analytical Methods of GMO Testing ... Definition: The range of concentrations over which the module performs in a linear manner with an. |
|
Definition of Minimum Performance Requirements for Analytical
13 oct. 2008 Analytical Methods of GMO Testing ... analytical method. ... Definition: The range of concentrations over which the method performs in a ... |
|
ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and
Each of these validation characteristics is defined in the attached Glossary. concentration (amounts) of analyte in the sample (including these ... |
|
Chapter 1 fundamental calculations in analytical chemistry
2 Problems of Instrumental Analytical Chemistry: A Hands-On Guide In general the concentration of a solution is defined as the. |
|
Guideline Bioanalytical method validation
21 juil. 2011 Acceptance criteria wider than those defined in this guideline may be used in ... concentrations by the analytical method reflect the ... |
|
Definition of micro and nanoplastics & analytical challenges
5 juin 2019 As the nature and concentrations of nanoplastics in the environment have not been measured yet we do not know anything about the importance of ... |
|
ICH guideline Q2(R2) on validation of analytical procedures
31 mars 2022 A linear relationship between analyte concentration and response should be evaluated across. 221 the working range of the analytical ... |
|
Guidance for the Validation of Analytical Methodology and
Validation and verification of analytical method. 9. For those qualitative methods with a pre-defined threshold concentration for report-. |
|
FDA
24 mai 2018 pharmacokinetic toxicokinetic |
|
CHAPTER 4 Calculations Used in Analytical Chemistry
Analytical molarity is the total number of moles of a solute, regardless of its chemical state, in 1 L of solution The analytical molarity describes how a solution of a |
|
Chapter 2
Table 2 1 Fundamental SI Units of Importance to Analytical Chemistry Measurement Both molarity and formality express concentration as moles of solute per liter of this definition makes an equivalent, and thus normality, a function of the |
|
Chapter 3: The Vocabulary of Analytical Chemistry
analysis we determine the identity, concentration, or properties of an ana- lyte A simple definition of a quantitative analytical method is that it is a mecha- |
|
Harmonised Guidelines for the Use of Recovery - Eurachem
types of analysis where loss of analyte during the analytical procedure is terms of the method per se and serves by definition as the only method for recovery is the ratio of the concentration of analyte found to that stated to be present |
|
Terminology in Analytical Measurement - Eurachem
measure, e g length, mass, time and concentration Validation and verification is another pair of words that have a changed definition in VIM 3 from what is |
|
Glossary of Analytical Terms
the series \Glossary of Analytical Terms" (GAT) in the journal Accreditation A sample whose analyte concentration is \Value consistent with the definition of |
|
Analytical chemistry
19 juil 2017 · A qualitative analysis determines the presence or absence of a particular compound, but not the mass or concentration By definition, qualitative |
|
GUIDELINES ON PERFORMANCE CRITERIA FOR METHODS OF
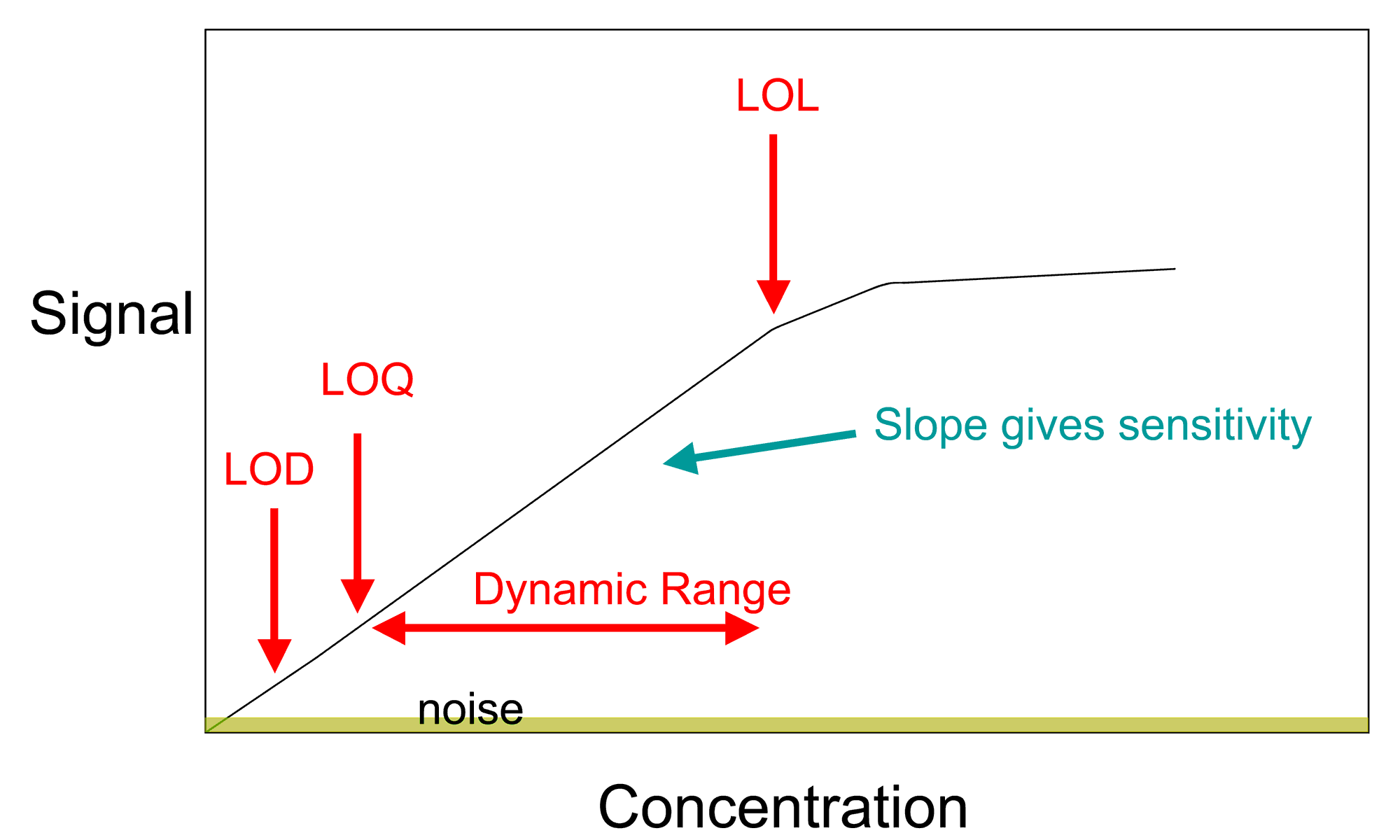
OECD Guidance Document on Pesticide Residue Analytical Methods, definition among analytical chemists, the LOQ is the concentration at which the average |
|
DEFINITION AND CLASSIFICATION OF INTERFERENCES IN
19 avr 2018 · ture a definition of interference is presented and recommendations are given concentration, causes a systematic error in the analytical result |