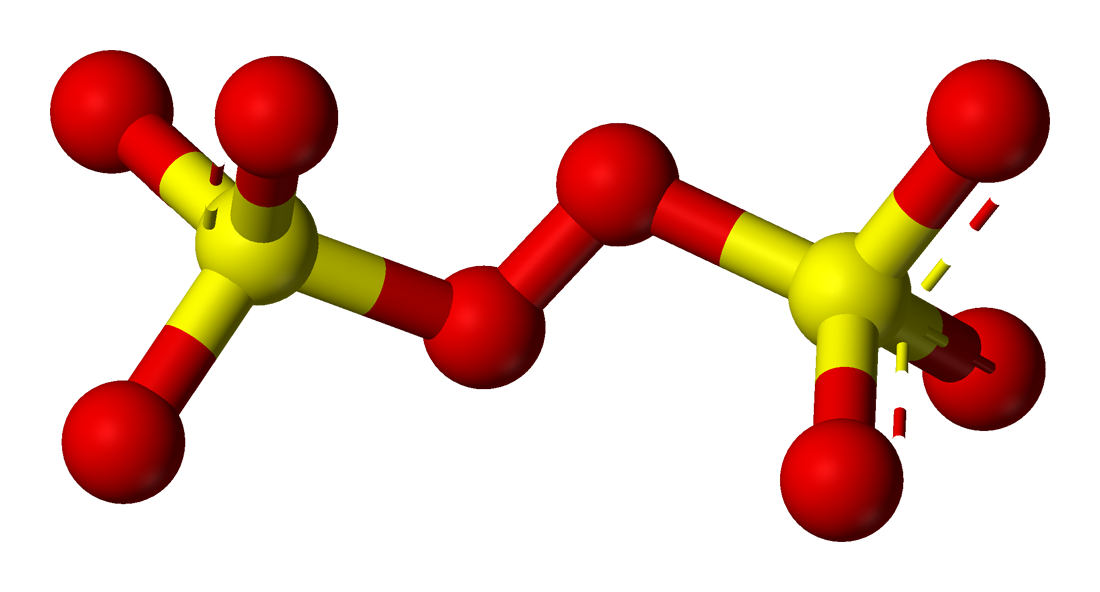
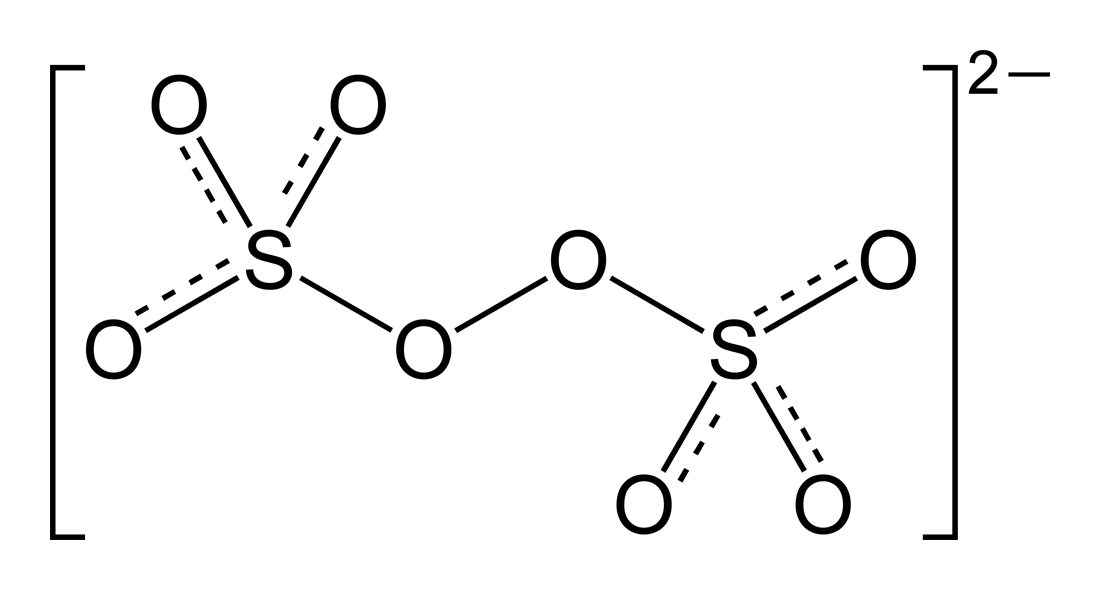
ion peroxodisulfate
|
A multifunctional potassium peroxodisulfate activation
desired Herein potassium peroxodisulfate (K 2 S 2 O 8) has been proposed as the eective activation agent for the synthesis of N S co-doped carbon nanosheets (N S-CNSs) from renewable onions Dierent from the other activation agents it was found that the K 2 S 2 O 8 |
|
Chimie VII
27 nov 2020 · On considère la réaction d'oxydation des ions iodure par les ions peroxodisulfate en solution aqueuse Cette réaction lente et totale met en |
- Description.
L'ion iodure, de formule I−, est un atome d'iode qui a gagné un électron afin d'avoir une couche externe d'électrons saturée (configuration 5s25p6).
Synthesis Process of N, S Co-Doped Carbon Nanosheets
All the chemicals were analytically pure and supplied by Shanghai Aladdin Bio-Chem Technology Co., Ltd, without further purification. Onion was purchased from the local market. After thoroughly washing with abundant deionized water to eliminate any contaminants, the peeled onion was dried at 80 °C for 12 h and then pulverized into a fine powder wit
Materials Characterization
The organic elemental quantitative analysis of the onion was performed on an elemental analyzer (Elementar vario el III, CHNS mode, German). The morphologies, microstructures, and element distribution of the products were observed by a scanning electron microscope (SEM, Zeiss Sigma 300, German) and a field emission transmission electron microscope
Electrochemical Measurements
The working electrodes are arranged by first grounding the as-obtained N, S-CNS-x (85 wt%), acetylene black (10 wt%), and polytetrafluoroethylene (PTFE, 5 wt%) with ethanol in an agate mortar to form a homogeneous slurry. Afterward, coat part of the slurry onto a round stainless-steel collector uniformly and then vacuum dry at 80 °C for 10 h in an
Synthesis Process of N, S Co-Doped Carbon Nanosheets
All the chemicals were analytically pure and supplied by Shanghai Aladdin Bio-Chem Technology Co., Ltd, without further purification. Onion was purchased from the local market. After thoroughly washing with abundant deionized water to eliminate any contaminants, the peeled onion was dried at 80 °C for 12 h and then pulverized into a fine powder wit
Materials Characterization
The organic elemental quantitative analysis of the onion was performed on an elemental analyzer (Elementar vario el III, CHNS mode, German). The morphologies, microstructures, and element distribution of the products were observed by a scanning electron microscope (SEM, Zeiss Sigma 300, German) and a field emission transmission electron microscope
Electrochemical Measurements
The working electrodes are arranged by first grounding the as-obtained N, S-CNS-x (85 wt%), acetylene black (10 wt%), and polytetrafluoroethylene (PTFE, 5 wt%) with ethanol in an agate mortar to form a homogeneous slurry. Afterward, coat part of the slurry onto a round stainless-steel collector uniformly and then vacuum dry at 80 °C for 10 h in an
|
Concentration en ions péroxodisulfate en fonction du temps (sans
L-1. • S2 : peroxodisulfate de sodium (2Na+(aq) + S2O8. 2-(aq)) de concentration molaire 80.10 |
|
TP N°3 : ETUDE CINETIQUE PAR DOSAGE DE LA
DES IONS PEROXODISULFATE ET DES IONS IODURES. Page 2. Classe de TS. TP N°3. Chimie. Prof. 2. I2 = f(t). 0. 0005. 0 |
|
Fiche de présentation et daccompagnement Niveau Terminale
8 juil. 2020 ) Déterminer la concentration initiale en ions peroxodisulfate dans le mélange et entrer sa valeur dans le programme Python (ligne 20). …………………… |
|
Concentration en ions péroxodisulfate en fonction du temps (sans
L-1. • S2 : peroxodisulfate de sodium (2Na+(aq) + S2O8. 2-(aq)) de concentration molaire 80.10 |
|
Teneur en fer dun produit sanitaire. La teneur en fer dun produit
Les ions peroxodisulfate comme les ions permanganate réagissent en solution aqueuse avec les ions Fe2+. Oxydation des ions Fe2+ en ion Fe3+. 1.2.Écrire les ... |
|
Chapitre VI: Lévolution des réactions chimiques
exemple: les ions iodure I - avec les ions peroxodisulfate S2O8. 2- état initial température Ti = 30°C espèce n°1: ions iodure. ✓ formule chimique: I. |
|
REACTION ENTRE LES IONS PEROXODISULFATE ET LES IONS
REACTION ENTRE LES IONS PEROXODISULFATE ET LES IONS IODURE-. CORRECTION. 1 ion iodure le mélange n'est pas stœchiométrique. 4) Les valeurs des quantités de ... |
|
TP 15 Ordre de réaction.docx
Réaction chimique entre les ions peroxodisulfate et les ions iodure. La réaction entre les ions peroxodisulfate et les ions iodure est modélisée par l |
|
Chimie VII
27 nov. 2020 ... ions iodure par les ions peroxodisulfate en solution ... - une solution de peroxodisulfate de sodium de concentration en ion peroxodisulfate. |
|
Catalyse
Montrer que l'ion Fe2+. (aq) catalyse l'oxydation de l'ion iodure par l'ion peroxodisulfate. De quel type de catalyse s'agit-il ? 12.5 Sens d'addition des |
|
REACTION ENTRE LES IONS PEROXODISULFATE ET LES IONS
1) Concentration initiale des ions peroxodisulfate dans le mélange réactionnel. 2) La réaction fournit du diiode(I2) et des ions sulfate (SO4. |
|
Réaction entre les ions peroxodisulfate et iodure
1) Concentration initiale des ions peroxodisulfate dans le mélange réactionnel. 2) La réaction fournit du diiode(I2) et des ions sulfate (SO4. |
|
Fiche de présentation et daccompagnement Niveau Terminale
Concentration en ions péroxodisulfate en fonction du temps 2) Déterminer la concentration initiale en ions peroxodisulfate dans le mélange et entrer sa ... |
|
Chapitre VI: Lévolution des réactions chimiques
exemple: les ions iodure I - avec les ions peroxodisulfate S2O8. 2- état initial température Ti = 30°C espèce n°1: ions iodure. ? formule chimique: I. |
|
Concentration en ions péroxodisulfate en fonction du temps (sans
On étudie la réaction d'oxydoréduction entre les ions iodures I- et les ions peroxodisulfate S2O8. 2-. ? Solutions aqueuses mise à disposition :. |
|
REACTION ENTRE LES IONS PEROXODISULFATE ET LES IONS
REACTION ENTRE LES IONS PEROXODISULFATE ET LES IONS IODURE. On mélange un volume V1 = 20 mL de peroxodisulfate d'ammonium (2NH4. |
|
Dosage fer(ii)-peroxodisulfate par titrimetrie thermo- metrique
Thermometric titrimetry is used to determine the enthalpy change and the rate con- stant for the reaction iron(II)-persulfate ion. |
|
TP 16 La chimie du suivi cinétique - Quelle grandeur permet de
potassium de concentration en ion iodure [I-] = 10 mol.L-1. Information 1 : Oxydation des ions iodure I- par les ions peroxodisulfate. |
|
CHIMIE ( 7 points)
réagissent avec les ions peroxodisulfate S2O8 -1 et d'un volume V2 = 10 mL de solution aqueuse de peroxodisulfate de potassium K2S2O8 de concentration ... |
|
Teneur en fer dun produit sanitaire. La teneur en fer dun produit
Les ions peroxodisulfate comme les ions permanganate réagissent en solution aqueuse avec les ions Fe2+. Un test avec une solution aqueuse d'hydroxyde de |
|
Réaction entre les ions peroxodisulfate et iodure
Réaction entre les ions peroxodisulfate et iodure 1) Concentration initiale des ions peroxodisulfate dans le mélange réactionnel |
|
TP cinétique 7 : action des ions peroxodisulfate sur les ions iodures :
On note Véq (t) le volume de solution de thiosulfate versé à l'équivalence pour chaque date t On cherchera l'ordre partiel par rapport aux ions peroxodisulfate |
|
REACTION ENTRE LES IONS PEROXODISULFATE ET LES IONS
REACTION ENTRE LES IONS PEROXODISULFATE ET LES IONS IODURE On mélange un volume V1 = 20 mL de peroxodisulfate d'ammonium (2NH4 |
|
CH08 TP cinétique
Exprimer en vous aidant du tableau que vous venez de compléter les quantités de matière en ions iodure peroxodisulfate et sulfate en fonction de la variable |
|
Mécanisme dune catalyse - ECEBacfr
On peut étudier la cinétique d'oxydation des ions iodure I?(aq) par les ions peroxodisulfate S2O8 2?(aq) en l'absence de catalyseur et à ? = 25 °C |
|
Concentration en ions péroxodisulfate en fonction du temps (sans
On étudie la réaction d'oxydoréduction entre les ions iodures I- et les ions peroxodisulfate S2O8 2- ? Solutions aqueuses mise à disposition : |
|
Oxydation des ions iodure - College Des Saints Coeurs Ain Najm
1 oct 2019 · Oxydation des ions iodure Les ions iodure (I - ) réagissent avec les ions peroxodisulfate (S2O8 2- ) selon une réaction lente et |
|
Chimie VII
27 nov 2020 · On considère la réaction d'oxydation des ions iodure par les ions peroxodisulfate en solution aqueuse Cette réaction lente et totale met en |
|
ÉTUDE DUNE CINÉTIQUE DE RÉACTION
I ] CINÉTIQUE DE LA RÉACTION DES IONS IODURE SUR LES IONS PEROXODISULFATE A°] Préparation des solutions 1°) Quelle masse de peroxodisulfate d'ammonium |
|
Chapitre VI: Lévolution des réactions chimiques - Physique - Chimie
La transformation chimique est le passage d'un système chimique de l'état initial à l'état final exemple: les ions iodure I - avec les ions peroxodisulfate |
| Investigating the rate of reaction between peroxydisulphate |
| Rate Properties of an Iodide Oxidation Reaction |
| Chem 112 – Experiment 3 – Simulation – Chemical Kinetics |
| Ion-pairing in aqueous solutions of peroxodisulfates - stubask |
| Experiment 5 Kinetics: The Oxidation of Iodide by Hydrogen |
| Searches related to ion peroxodisulfate filetype:pdf |
How do you convert peroxodisulfate to molecular iodine?
- The Peroxodisulfate Iodide Clock Reaction (PODS/I{reaction) In this reaction, two iodide ions provide one electron each to reduce the peroxodisulfate ion, S 2O 8 2{, to form two stable sulfate ions and molecular iodine.
What is the peroxodisulfate iodide clock reaction?
- The Peroxodisulfate Iodide Clock Reaction (PODS/I{reaction) In this reaction, two iodide ions provide one electron each to reduce the peroxodisulfate ion, S 2O 8 2{, to form two stable sulfate ions and molecular iodine.
. The overall redox reaction is shown below.
. S 2O 2 8(aq) + 2 I (aq) I
What is the catalyst for the reaction of iodide with hydrogen peroxide?
- An effective catalyst exists for the reaction of iodide with hydrogen peroxide.
. The catalyst is iron (II) ions dissolved in the reaction mixture.
. By adding these ions to the reaction mixture we can increase the rate of the reaction.
. Eye protection must be worn throughout this experiment.
What is the rate law for hydrogen peroxide iodine?
- 22 2 2 hydrogenperoxide iodine. q) + H O (aq) I (s) + 2H O(l)?.
. The rate law for this reaction should include the concentrations of iodide, hydrogen ion, and hydrogen perioxide.
. However, if the concentration of H+ is held constant throughout the experiment then its effect will not appear in the rate law.
|
TP N°3 : ETUDE CINETIQUE PAR DOSAGE DE LA - Physagreg
DES IONS PEROXODISULFATE ET DES IONS IODURES La réaction entre le diiode et les ions thiosulfate est rapide et permet le titrage du diiode, l'utilisation |
|
Reaction entreles ions peroxodisulfate et iodure
Réaction entre les ions peroxodisulfate et iodure 1) Concentration initiale 2) La réaction fournit du diiode(I2) et des ions sulfate (SO4 2-) Ecrire l'équation |
|
Action des ions peroxodisulfate sur les ions iodures - Pages perso
TP7 : cinétique de la réaction de l'ion iodure sur les ions peroxodisulfates température ambiante entre les ions iodure I- et les ions peroxodisulfate S2O8 |
|
Couleur et cinétique - Jeulin
PEROXODISULFATE SUR LES IONS IODURE obtenir une solution aqueuse de concentration molaire en ions peroxodisulfate [S2O8 2-]Init = 1,20 x 10-2 mol |
|
CHIMIE - Université de Monastir
réagissent avec les ions peroxodisulfate S2O8 2- L'équation associée à -1 et d'un volume V2 = 10 mL de solution aqueuse de peroxodisulfate de potassium |
|
TP16 La chimie du suivi cinétique
potassium de concentration en ion iodure [I-] = 1,0 mol L-1 Information 1 : Oxydation des ions iodure I- par les ions peroxodisulfate On considère la réaction |
|
TM2 - Evolution temporelle des systèmes chimiques I
I Décomposition de l'ion peroxodisulfate Les ions peroxodisulfate S2O8 Ecrire l'équation de la réaction associée, sachant que l'on forme des ions sulfate |
|
ER Cinétique Chimique Cinétique avec prél`evement et dosage
5) Écrire l'équation bilan du dosage de l'iode par l'ion thiosulfate D'o`u les concentration initiales en ions iodure et en ions peroxodisulfate dans le mélange : |
|
Entraînement de cinétique chimique PCSI - CPGE Brizeux
Spectrophotométrie On se propose de déterminer l'ordre et la constante de vitesse de la réaction d'oxydation des ions iodure I– par les ions peroxodisulfate |