butanol formule
What is the chemical equation butanol?
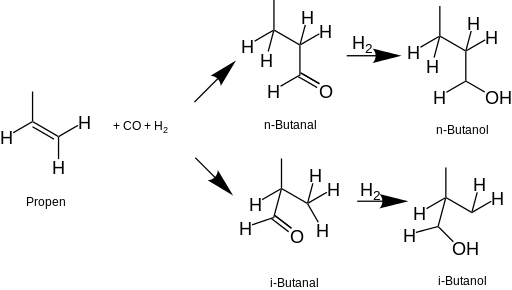
Butanol (also called butyl alcohol) is a four-carbon alcohol with a formula of C 4 H 9 O H, which occurs in five isomeric structures (four structural isomers), from a straight-chain primary alcohol to a branched-chain tertiary alcohol; all are a butyl or isobutyl group linked to a hydroxyl group (sometimes represented as BuOH, n-BuOH, i-BuOH, and t-BuOH).
What is the structural formula of butanol?
Butanol (also called butyl alcohol) is a four-carbon alcohol with a formula of C 4 H 9 O H, which occurs in five isomeric structures (four structural isomers), from a straight-chain primary alcohol to a branched-chain tertiary alcohol; all are a butyl or isobutyl group linked to a hydroxyl group (sometimes represented as BuOH, n-BuOH, i-BuOH, and t-BuOH).
What is the difference between butanol and isobutanol?
n-Butanol has a straight-chain structure with the alcohol at the terminal carbon. Sec-butanol is also a straight-chain molecule but the OH group is attached to an internal carbon. Isobutanol is a branched isomer with the OH group at the terminal carbon, while tert-butanol refers to the branched isomer with the OH group at an internal carbon.
How can be produced butanol from butanediol?
Butanol can be produced by fermentation employing a number of microorganisms such as Clostridium acetobutylicum and Clostridium beijerinckii. The typical ratio of ABE in the final product is usually 3:6:1 with maximum concentration of total solvents (ABE) being 20 g l −1 when using traditional strains and traditional batch fermentation process.

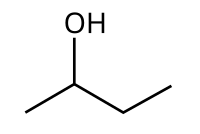
Molecular and Structural Formula for n-Butanol (1-Butanol)

Structural Formula for 1-Butanol (C4H9OH)

Complete Combustion of Butanol (C4H9OH) Balanced Equation
|
1-Butanol - INRS
1 mar 2010 · Formule C4H10O Numéro CAS 71-36-3 Famille chimique Alcool aliphatique Formule éclatée Synonymes Alcool butylique ; n-Butanol |
|
BUTANOL-1 - SORDALAB
30 nov 2018 · SECTION 3: Composition/informations sur les composants 3 1 Substances Synonymes : Alcool butylique primaire Formule : C4H10O |
|
Butanol-1 995% AGR
11 nov 2016 · butane-1-ol n-butanol Numéro index : 603-004-00-6 Numéro CE : 200-751-6 n° CAS : 71-36-3 Code du produit : BUTL-10A Formule brute |
|
Fiche de Données de Sécurité: Butanol-1 - Carl Roth
Substances Nom de la substance Butanol-1 Formule moléculaire C?H??O Masse molaire 7412 g/mol No d'enreg REACH 01-2119484630-38-xxxx |
|
40808 1-Butanol 995% - Laurylab
L'inhalation de vapeurs peut provoquer somnolence et vertiges 3 Composition/Information des composants Dénomination: 1-Butanol Formule: CH3(CH2)3OH M =74 |
|
Tert-Butanol - Chimie Plus
Tert-Butanol Pour analyses Ref: 24694 CAS No 75-65-0 P M : 74 12 Formule C4 H10 O Point éclair 11°C Apparence Liquide clair Couleur = |
|
Butan-1-ol - INRS
Numéro index 603-004-00-6 Synonymes Alcool butylique n-Butanol Caractéristiques Utilisations Edition Mise à jour 2011 Formule : CH -CH -CH -CH OH |
|
Butan-1-ol - INRS
Formule Chimique Nom Numéro CAS Numéro CE Numéro index Synonymes C H O Butan-1-ol 71-36-3 200-751-6 603-004-00-6 Alcool butylique n-Butanol |
|
BUTANOL-1 - SORDALAB
30 nov 2018 · BUTANOL-1 / SI019 BUTANOL-1 SI019 1L - SI019 2 5L FICHE DE DONNÉES DE SÉCURITÉ Formule : C4H10O Poids moléculaire : 7412 g/mol |
|
Fiche de Données de Sécurité: Butanol-1 - Carl Roth
RUBRIQUE 3: Composition/informations sur les composants 3 1 Substances Nom de la substance Butanol-1 Formule moléculaire C?H??O Masse molaire |
|
Butanol-1 CAS 71-36-3 101988
MDA_CHEM-101988.pdf |
|
N-Butanol - Oxoplast
Butan-1-ol n-Butanol - en allemand : d/ formule chimique Le n-butanol est un liquide transparent incolore présentant une odeur caractéristique |
|
Identification disomères (22 points) 1) (4 pts) Formules semi
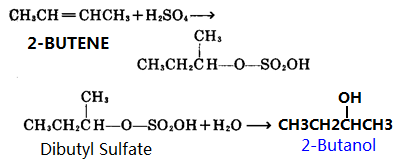
C est un alcool secondaire (cétone obtenue lors de son oxydation) de formule brute C4H10O : C est le butan-2-ol 7) (6 pts) Equation de la réaction d'obtention |
|
Butan-1-ol - Wikipédia
Le butan-1-ol ou n-Butanol est un alcool primaire de formule semi-développée CH3-(CH2)3-OH Il a pour isomères l'isobutanol le butan-2-ol et le |
|
Stéréro chimie
Exercice 6 : recherche d'isomères du butanol 1 Représenter la formule semi-développée de tous les alcools de formule brute C4H10O |
|
40808 2-Butanol - Laurylab
3 Composition/Information des composants Dénomination: 2-Butanol Formule: C4H10O M =7412 CAS [78-92-2] Numéro CE (EINECS): 201-158-5 |
| N-BUTANOL - Apurv Chemicals |
| -Butanol 10% w/w 3 Product Specification - Sigma-Aldrich |
| Combustion of butanol equation - Bleu Wing Investigations |
| Butanol: A Second Generation Biofuel - University of Illinois |
| Material Safety Data Sheet - MiraCosta College |
| Searches related to butanol formule filetype:pdf |
What is the chemical equation butanol?
- Butanol (also called butyl alcohol) is a four-carbon alcohol with a formula of C 4 H 9 O H, which occurs in five isomeric structures (four structural isomers), from a straight-chain primary alcohol to a branched-chain tertiary alcohol; all are a butyl or isobutyl group linked to a hydroxyl group (sometimes represented as BuOH, n-BuOH, i-BuOH, and t-BuOH).
What is the structural formula of butanol?
- Butanol (also called butyl alcohol) is a four-carbon alcohol with a formula of C 4 H 9 O H, which occurs in five isomeric structures (four structural isomers), from a straight-chain primary alcohol to a branched-chain tertiary alcohol; all are a butyl or isobutyl group linked to a hydroxyl group (sometimes represented as BuOH, n-BuOH, i-BuOH, and t-BuOH).
What is the difference between butanol and isobutanol?
- n-Butanol has a straight-chain structure with the alcohol at the terminal carbon.
. Sec-butanol is also a straight-chain molecule but the OH group is attached to an internal carbon.
. Isobutanol is a branched isomer with the OH group at the terminal carbon, while tert-butanol refers to the branched isomer with the OH group at an internal carbon.
How can be produced butanol from butanediol?
- Butanol can be produced by fermentation employing a number of microorganisms such as Clostridium acetobutylicum and Clostridium beijerinckii.
. The typical ratio of ABE in the final product is usually 3:6:1 with maximum concentration of total solvents (ABE) being 20 g l ?1 when using traditional strains and traditional batch fermentation process.
|
1-Butanol - INRS
1 mar 2010 · Famille chimique Alcool aliphatique Formule éclatée Synonymes Alcool butylique ; n-Butanol Names / Synonyms 1-Butanol ; Butyl alcohol ; |
|
BUTANOL-1 - SORDALAB
30 nov 2018 · SECTION 3: Composition/informations sur les composants 3 1 Substances Synonymes : Alcool butylique primaire Formule : C4H10O |
|
METHYL-3-BUTANOL-1 - SORDALAB
Formule : C5H12O Poids moléculaire : 88 15 g/mol N° CAS: 123-51-3 N° CE: 204-633-5 N° Index: 603-006-00-7 Composants dangereux selon Règlement |
|
Tert-Butanol 99% AGR
14 nov 2016 · BUTL-T0A Formule brute : (CH3)3COH 1 2 Utilisations identifiées pertinentes de la substance ou du mélange et utilisations déconseillées |
|
Fiche de Données de Sécurité: Butanol-2 - Carl Roth
Numéro d'enregistrement (REACH) 01-2119475146-36-xxxx Numéro CE 201- 158-5 Numéro CAS 78-92-2 Formule moléculaire C₄H₁₀O Masse molaire |
|
Fiche de Données de Sécurité - Restek
03-07-2018 08-06-2017 FR/FR 1-Butanol page 1 de 14 Formule moléculaire: CH3OH Famille chimique: Estimations de toxicité aiguë n-Butyl alcohol 5 |