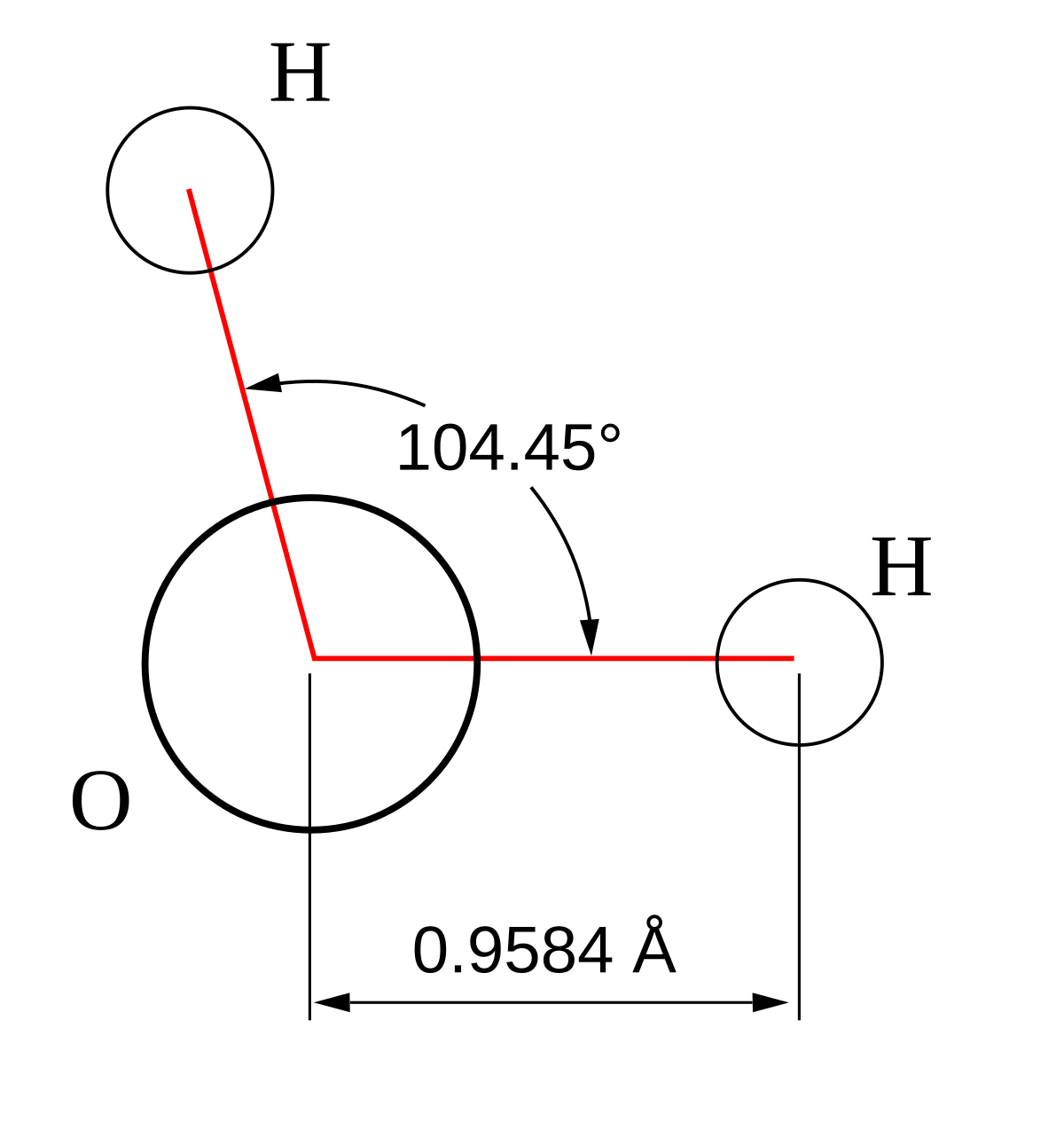
representation molecule (Lewis, valence)
|
Revised 12/2015 Chemistry 1104 L LEWIS STRUCTURES
The purpose of this experiment is to gain practical experience of drawing lewis structures and to use molecular models to represent the three-dimensional shapes of molecules which will lead to a better understanding of the concepts of covalent bonding and molecular structure |
|
Lecture B5 Valence Bond Theory
set of empirical rules for predicting a molecular geometry using as input a correct Lewis Dot representation Valence Bond theory more advanced description of orbitals in molecules We emphasize just one aspect of this theory: Hybrid atomic orbitals Works especially well for organic molecules Molecular Orbital theory |
|
Electron Dot (Lewis) Structures
A Lewis or Electron Dot Structure is a convenient representation of the valence electrons in an atom An electron dot structure for an atom is simply the symbol for the element surrounded by a number of dots equal to the number of valence electrons |
|
Chem 1020 Laboratory Molecular Models: Lewis Structure and
1 Lewis structure is commonly used to describe how valence electrons are distributed and what bonds are formed between atoms within a molecule Lewis structure is a fundamental representation of a molecule and it reveals its both chemical reactivity and physical properties such as solubility melting point and boiling point |
How many valence electrons are in a Lewis structure?
All the valence electrons of the atoms in a Lewis structure must appear in the structure. For the A-group elements, the number of valence electrons of an atom is equal to the group number. For example, carbon, in Group 4A, has 4 valence electrons. The number of unpaired electrons on an atom of Groups 4A through 8A is 8 minus the group number.
What does the Lewis dot represent in ethylene?
...and it gives us information about molecular geometry that was missing in the Lewis Dot structures. The Lewis Dot representation of ethylene tells us nothing about the relative orientation of the hydrogens, located in trigonal orbitals about each carbon. For example:
What is a Lewis structure in chemistry?
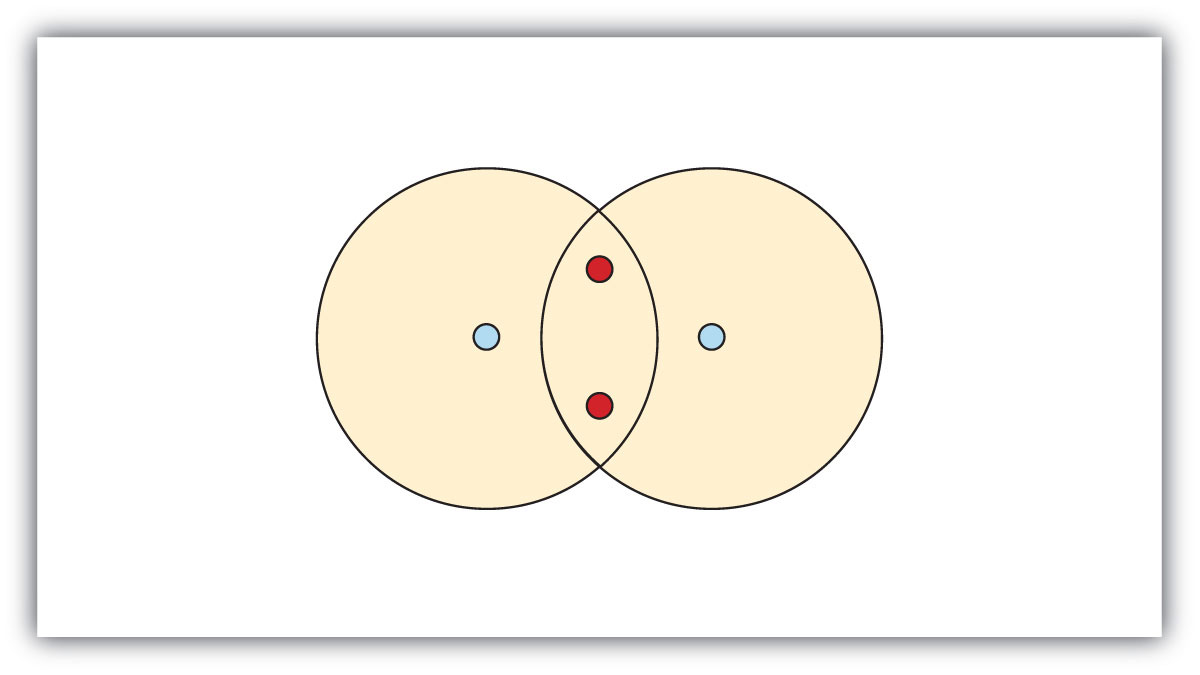
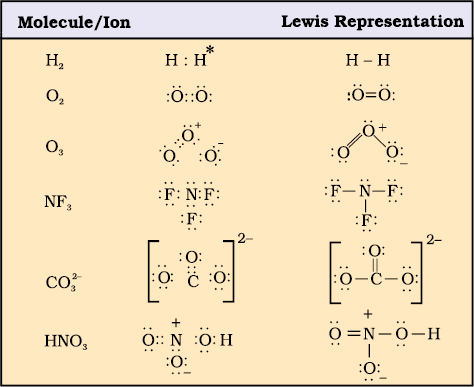
Lewis structure is a representation of covalent bonding where shared electron pairs are shown as lines and lone electron pairs are shown as dots. When drawing a Lewis structure, the octet rule is followed to attain the most stable electron configuration and achieve a complete octet of electrons for each atom in the molecule.
What is the purpose of a Lewis Structure Experiment?
LEWIS STRUCTURES The purpose of this experiment is to gain practical experience of drawing lewis structures and to use molecular models to represent the three-dimensional shapes of molecules, which will lead to a better understanding of the concepts of covalent bonding and molecular structure.
|
Représenter les couples acide-base
valence (couche externe) des atomes qui la constituent. ? Le schéma de Lewis d'une molécule consiste à représenter tous les doublets. |
|
Structures de lewis.pdf
La structure de Lewis consiste à définir l'allocation des électrons sur ou entre les atomes de la molécule. Seuls les électrons de valence sont considérés. |
|
Schéma de Lewis
L'atome d'hydrogène possède 1 électron de valence et cherche à atteindre la configuration Représentation de la molécule avec des liaisons simples. |
|
Fiche 2: Représentation de Lewis des espèces chimiques
La représentation de Lewis d'une molécule fait apparaitre (1) On détermine le nombre d'électrons de valence dans l'atome isolé (grâce au schéma de Lewis. |
|
1 Théorie de Lewis
On termine la représentation de Lewis en entourant l'édifice avec Le nombre total d'électrons de valence de cette molécule est. |
|
Modèle de Lewis Modèle de Lewis
27 oct. 2017 1.a - Proposer une représentation de Lewis de chaque espèce ... La molécule de monoxyde de carbone est constituée d'un atome d'oxygène (Z ... |
|
Aucun titre de diapositive
permettant de représenter les entités L'excitation d'un atome pour augmenter sa valence n'est ... Schéma de Lewis moléculaire " cases quantiques. |
|
Atomes & molécules CORRIGE
31 janv. 2019 P possède 5 électrons de valence et S en possède 6. Les deux schémas de Lewis ... Représenter le schéma de Lewis de la molécule de dichlore. |
|
Le Modèle de LEWIS
La valence normale d 'un élément se déduit du schéma de Lewis atomique et Construction du schéma de Lewis moléculaire ... représenter la même molécule. |
|
La représentation de Lewis Les atomes sont les éléments constitutifs
Une représentation des atomes et molécules : la représentation de Lewis l'élément chimique. Atomes Electrons de valence. Représentation de. Lewis. |
Comment savoir la valence d'une molécule ?
. On aperçoit au passage que les symboles ne sont pas disposés n'importe comment mais dans des colonnes avec à leur tête des chiffres romains.
Comment faire la représentation de Lewis d'une molécule ?
Comment savoir quels sont les électrons de valence ?
Comment définir la valence d'un atome ?
. Dans une molécule ou un ion, la valence d'un atome est le nombre de liaisons covalentes que cet atome a formées.
|
LES MOLECULES: REPRESENTATION CORRECTION - Educonline
I REPRESENTATION DE LEWIS DES MOLECULES 1) Les atomes • Les atomes sont représentés conventionnellement par des boules de couleur A chaque |
|
Modèle de Lewis Modèle de Lewis - Étienne Thibierge
27 oct 2017 · 7 - Proposer une représentation de Lewis possible pour la molécule de monoxyde de Cl : 1s2 2s2 2p6 3s2 3p5 donc 7 électrons de valence ; |
|
Le Modèle de LEWIS - BIENVENUE SUR LA PAGE DE THIERRY
molécule - Elle correspond en général au nombre d'électrons célibataires de l' atome considéré - La valence normale d 'un élément se déduit du schéma |
|
CH7 – CM2 – Structure des Molécules Structure des Molécules
Représentation de Lewis Pour illustrer la manière dont chaque atome va interagir avec ses voisins, on représente les électrons de sa couche de valence : |
|
Architecture de la matière - Chimie en PCSI
les représentations des molécules sont planes, et de la méthode VSEPR, Le schéma de Lewis d'un atome ne fait apparaître que ses électrons de valence, |
|
CORRIGE - Chimie en PCSI
de*valence*des*atomes*associés*par*liaison*covalente ** Dans*le*cas*d'un* ion Cherchons*le*schéma*de*Lewis*de*la*molécule*SF4*:** 1) Donner* les * représentations* de* Lewis* des* trois* isomères* du* dichloroéthène,* de* |
|
EXERCICES - Physicus
Établir le schéma de Lewis de molécules et d'ions mono ou poly valence des atomes présents dans cette molécule c En déduire le nombre de doublets formés puis vérifier s'il Représenter les schémas de Lewis des gaz nobles hélium |
|
Schéma de Lewis des molécules et structures spatiales
Electrons de la couche de valence : - Les électrons sont répartis en couche autour du noyau de l'atome - La dernière couche sur laquelle se trouvent des |








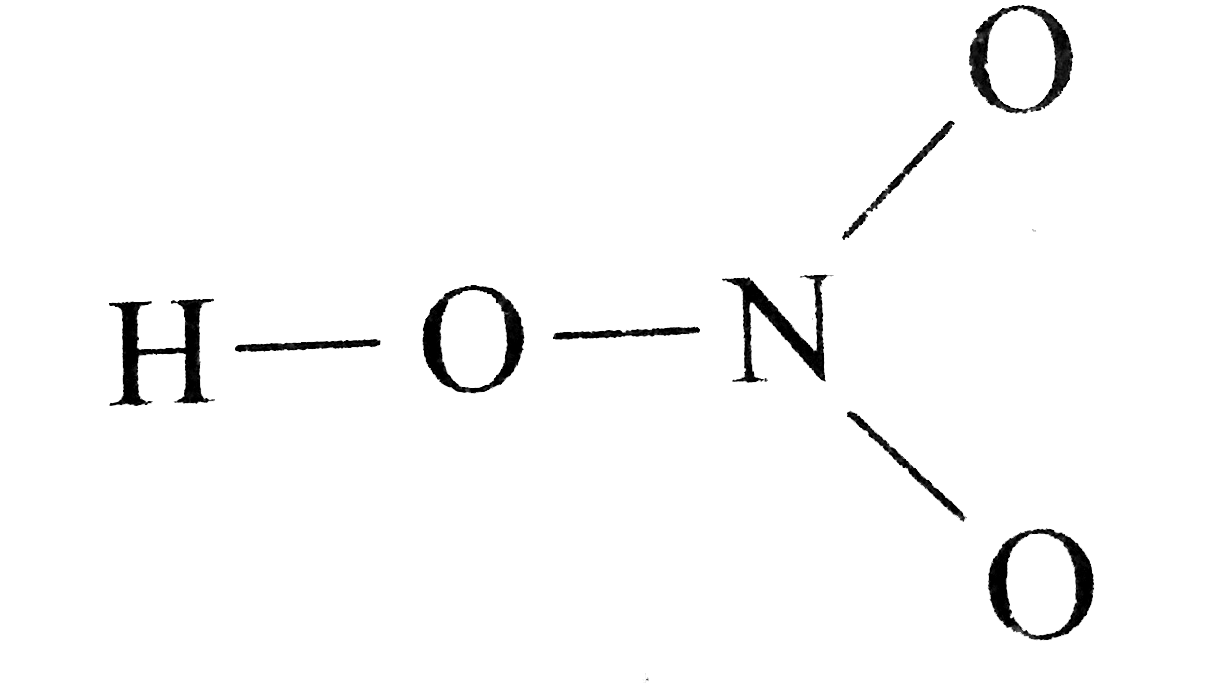



![Chemical bonding and molecular structure grade 11 - [PDF Document] Chemical bonding and molecular structure grade 11 - [PDF Document]](https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/1941/2017/05/30162552/Methane.png)



![33a VSEPR Theorypdf - [PDF Document] 33a VSEPR Theorypdf - [PDF Document]](https://www.webassign.net/question_assets/wertzcams3/ch_6/images/figure6-10.png)