electrochemistry class 12 notes pdf download
|
Chemistry Notes for Class 12 Chapter 3 Electrochemistry
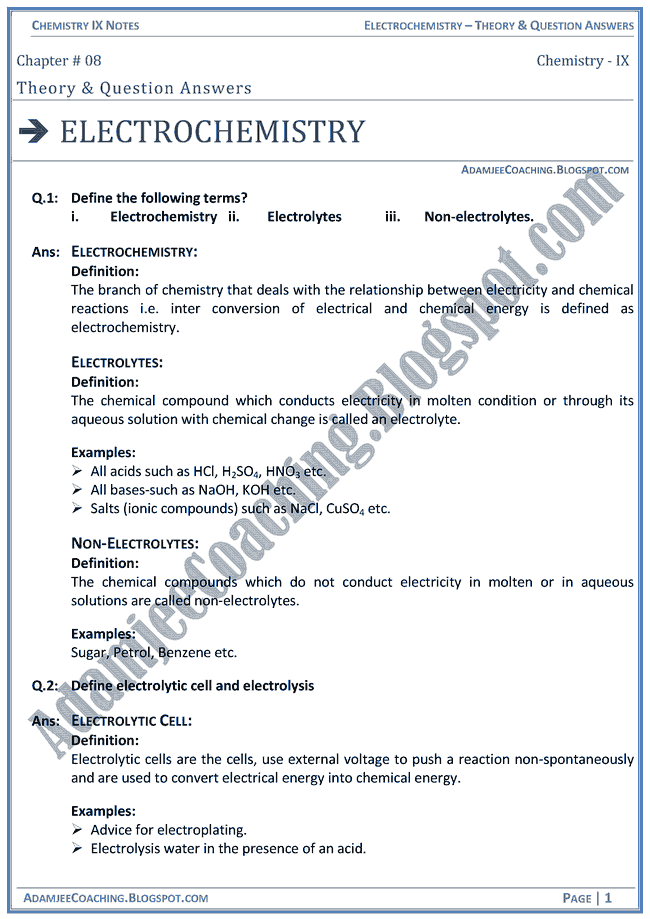
Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations Importance of Electrochemistry Production of metals like Na Mg Ca and Al Electroplating |
|
ElectrElectrochemistrochemistryy
After studying this Unit you will be able to describe an electrochemical cell and differentiate between galvanic and electrolytic cells; apply Nernst equation for calculating the emf of galvanic cell and define standard potential of the cell; derive relation between standard potential of the cell Gibbs energy of cell reaction and its equilibrium |
|
CHEM-1100 Chapter 19 Lecture Notes
Introduction Electrochemistry deals with interconversion of electrical and chemical energy Many chemical changes can be clearly related to the electrons that move from one species to another Often this electron exchange can be captured to do electrical work external to the chemical system (storage battery fuel cell) |
Where can I find electrochemistry class 12 notes?
The Selfstudys website provides the Electrochemistry class 12 notes in a PDF so that students can easily access it. All students should have easy and free access to the class 12 Chemistry notes so that they don’t need to search for here and there.
What is electrochemistry in chemistry?
Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. Production of metals like Na, Mg. Ca and Al. Electroplating. Purification of metals.
How to create a well structured electrochemistry class 12 chemistry notes?
The process of creating a well structured Electrochemistry Class 12 Chemistry Notes starts with understanding the Class 12 Chemistry Syllabus. After understanding the Syllabus experts refer to the prescribed NCERT Class 12 Chemistry textbooks to begin creating the revision notes of Electrochemistry.
Why are the Class 12 chemistry notes provided in PDF?
Provided in the PDF: The class 12 Electrochemistry notes are provided in the PDF as it helps students to solve their doubts then and there. All Topics are Covered: In the class 12 Chemistry notes, all topics of the chapter Electrochemistry according to the Class 12 Chemistry Syllabus.
Objectives
After studying this Unit, you will be able to describe an electrochemical cell and differentiate between galvanic and electrolytic cells; apply Nernst equation for calculating the emf of galvanic cell and define standard potential of the cell; derive relation between standard potential of the cell, Gibbs energy of cell reaction and its equilibrium
3.3 Nernst Equation
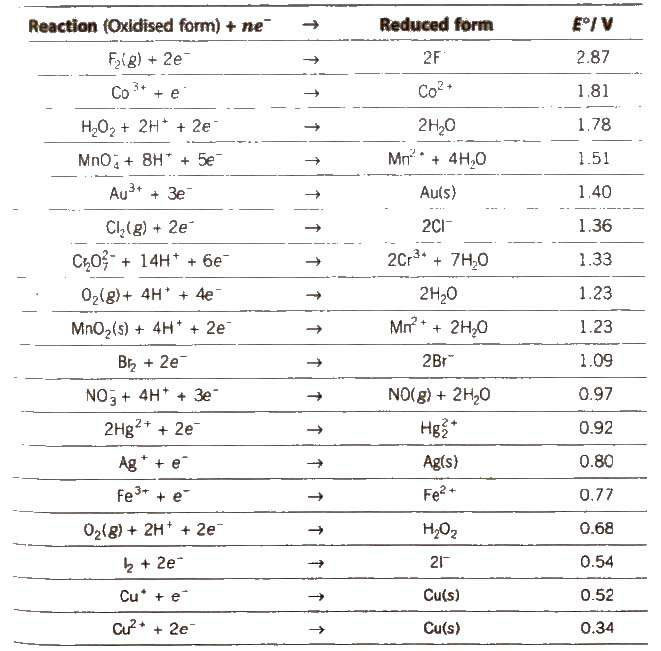
We have assumed in the previous section that the concentration of all the species involved in the electrode reaction is unity. This need not be always true. Nernst showed that for the electrode reaction: Mn+(aq) + ne–→ M(s) the electrode potential at any concentration measured with respect to standard hydrogen electrode can be represented by: ) = (
E ( E [M]
n + /M n + ) – ln M M /M nF [M n+ ] but concentration of solid M is taken as unity and we have ncert.nic.in
3.4.1 Measurement of the Conductivity of Ionic Solutions
We know that accurate measurement of an unknown resistance can be performed on a Wheatstone bridge. However, for measuring the resistance of an ionic solution we face two problems. Firstly, passing direct current (DC) changes the composition of the solution. Secondly, a solution cannot be connected to the bridge like a metallic wire or other solid
Quantitative Aspects of Electrolysis
Michael Faraday was the first scientist who described the quantitative aspects of electrolysis. Now Faraday’s laws also flow from what has been discussed earlier. ncert.nic.in
3.5.1 Products of Electrolysis
Products of electrolysis depend on the nature of material being electrolysed and the type of electrodes being used. If the electrode is inert (e.g., platinum or gold), it does not participate in the chemical reaction and acts only as source or sink for electrons. On the other hand, if the electrode is reactive, it participates in the electrode reac
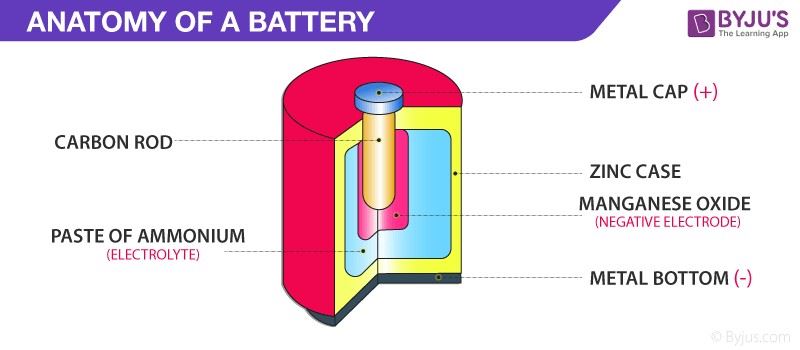
3.6 Batteries
Any battery (actually it may have one or more than one cell connected in series) or cell that we use as a source of electrical energy is basically a galvanic cell where the chemical energy of the redox reaction is converted into electrical energy. However, for a battery to be of practical use it should be reasonably light, compact and its voltage s
The Hydrogen Economy
At present the main source of energy that is driving our economy is fossil fuels such as coal, oil and gas. As more people on the planet aspire to improve their standard of living, their energy requirement will increase. In fact, the per capita consumption of energy used is a measure of development. Of course, it is assumed that energy is used for
Summary
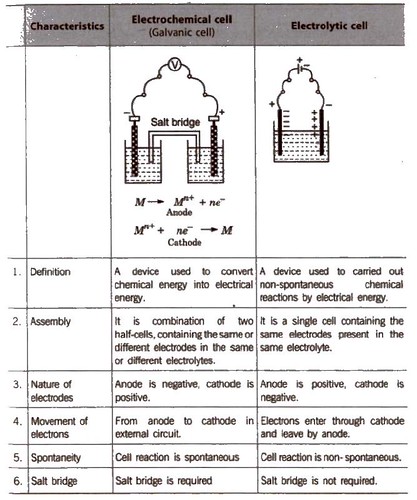
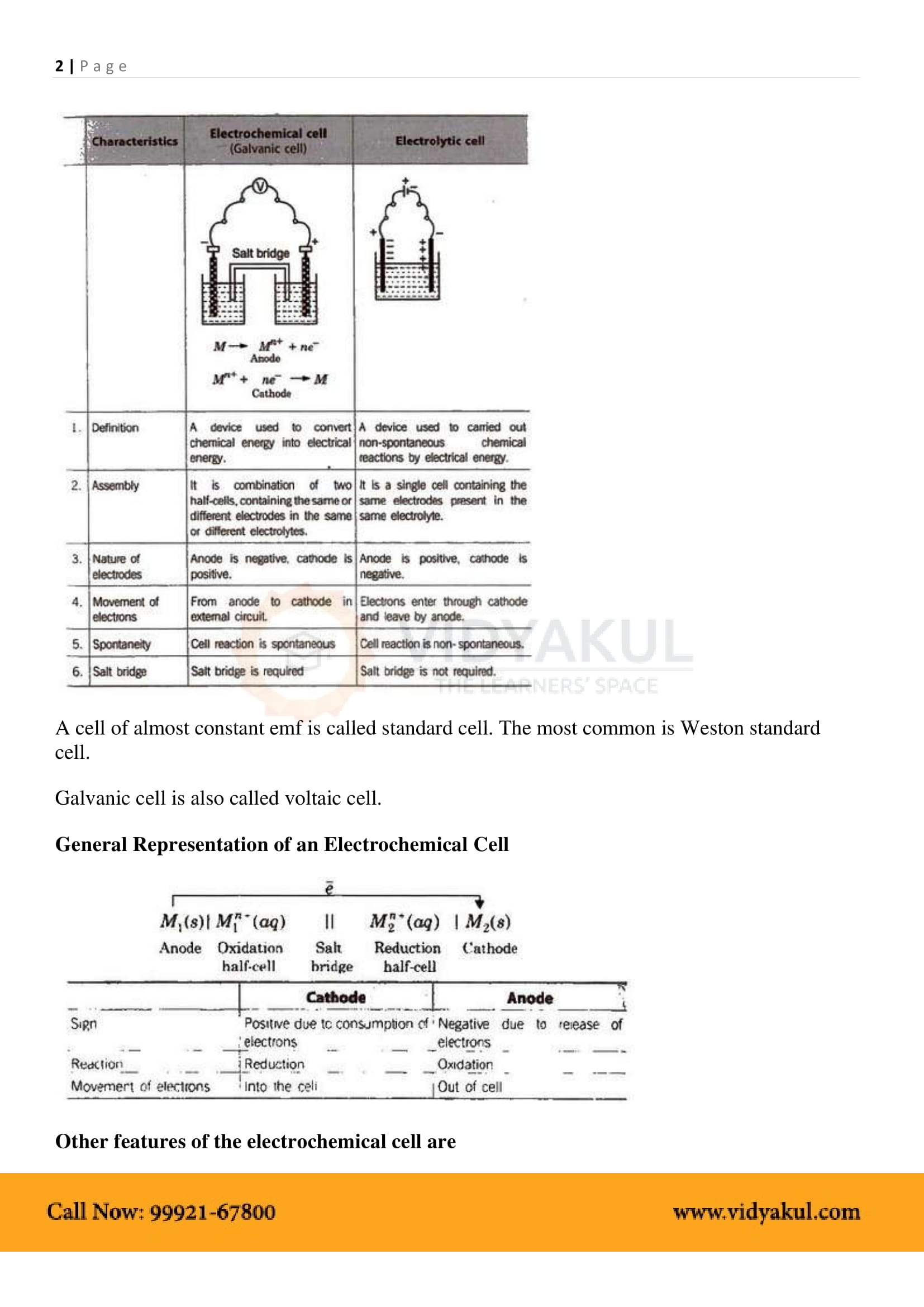
An electrochemical cell consists of two metallic electrodes dipping in electrolytic solution(s). Thus an important component of the electrochemical cell is the ionic conductor or electrolyte. Electrochemical cells are of two types. In galvanic cell, the chemical energy of a spontaneous redox reaction is converted into electrical work, whereas in an
( (ÄrGV cell ) and equilibrium constant
) = – RT ln K) of the reaction taking place in the cell. Concentration dependence of the potentials of the electrodes and the cells are given by Nernst equation. The conductivity, κ, of an electrolytic solution depends on the concentration of the electrolyte, nature of solvent and temperature. Molar conductivity, Ëm, is defined by = κ/c where c is
|
Chemistry Notes for class 12 Chapter 3 Electrochemistry .pdf
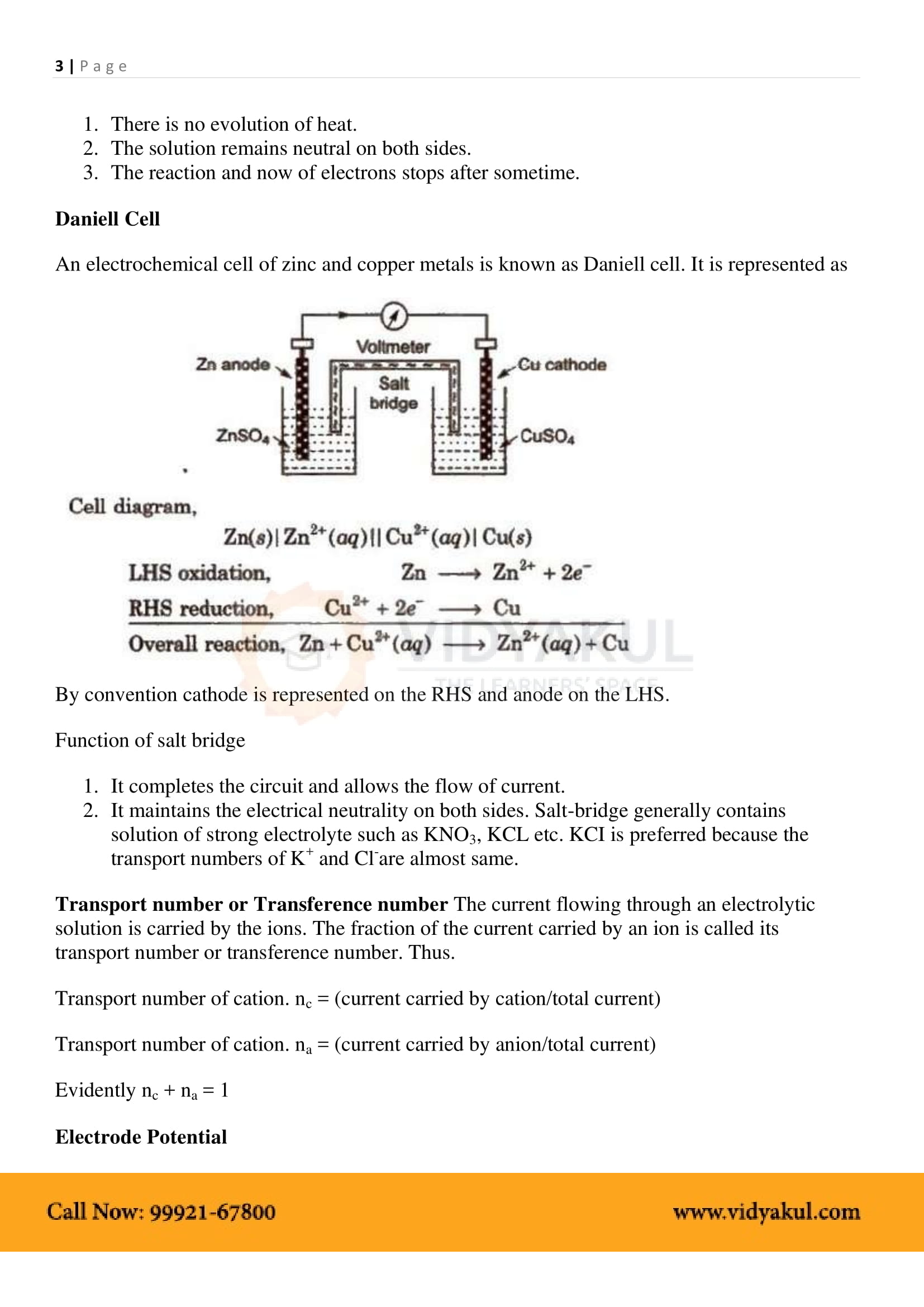
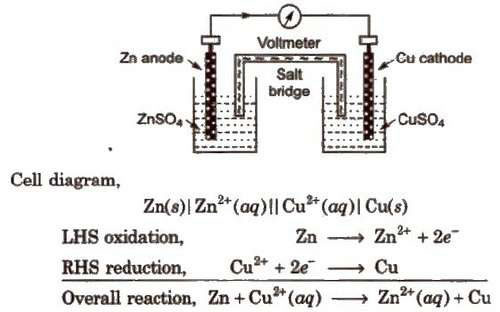
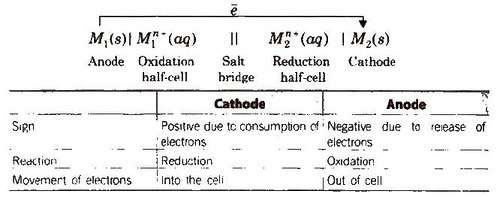
By convention cathode is represented on the RHS and anode on the LHS. Function of salt bridge. 1. It completes the circuit and allows the flow of current. |
|
Electrochemistry Electrochemistry
As mentioned earlier (Class XI Unit 8) a galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous redox reaction into |
|
Electrochemistr ochemistr ochemistry Electrochemistr ochemistr
65 Electrochemistry. As mentioned earlier (Class XI Unit 8) a galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous. |
|
ASPIRATIONS Institute
Further show : (a) Which of the electrode is negatively charged ? For more FREE DOWNLOADS visit www.aspirationsinstitute.com. Page 14. 30 |
|
Dr.Pusalkar Notes ELECTROCHEMISTRY S.Y.B.Sc
xii)Single Electrode Potential: A difference of electrical potential developed between the metal electrode and its surrounding salt solution at equilibrium is |
|
CBSE Class 12 Chemistry Deleted Syllabus Portion for 2020-21
12. Aldehydes. Ketones and. Carboxylic CBSE Class 12 Chemistry Syllabus ... D. Electrochemistry Variation of cell potential in Zn/Zn 2+ |
|
TOPIC 6: ELECTROCHEMISTRY
12 – topic 6: electrochemistry. Grade 12 Chemistry • Topic 6: Electrochemistry. SUGGESTIONS FOR INSTRUCTION. TEachEr NoTEs historical Development of Voltaic |
|
Formulae For ELECTROCHEMISTRY
12. ?. ? m x1000. = C. Remember: Unit of ?m in above formula is Scm2mol-1 1-?. XII Chemistry. CHAPTER 3 - ELECTROCHEMISTRY. |
|
ELECTROCHEMISTRY
ELECTROCHEMISTRY. 1. The standard electrode potential E 12. A solution of Ni(NO3)2 is electrolysed between platinum electrodes using 0.1 Faraday. |
|
General Chemistry II Chapter 19 Lecture Notes Electrochemistry
Electrochemistry deals with interconversion of electrical and chemical energy. Automotive 6V and 12V (soon 42V?) batteries are built from multiple cells ... |
|
Chemistry Notes for class 12 Chapter 3 Electrochemistry - Ncert Help
The reaction and now of electrons stops after sometime Daniell Cell An electrochemical cell of zinc and copper metals is known as Daniell cell It is represented |
|
Electrochemistry - Mahesh Tutorials Science
Electrochemistry is the study of production of electricity Electrochemical Cells are of two types: electrolyte with dilution is mainly due to [CBSE AIPMT] |
|
Xii Electrochemistry Notes Class 12
Free PDF Download 12th Class Chemistry Notes Chapter 4 Chemistry NCERT CBSE Syllabus Class 12 Notes Study Electrochemistry class 12 cbse pdf |
|
Electrochemistry - NCERT
As mentioned earlier (Class XI, Unit 8) a galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous redox reaction into electrical |
|
Xii Electrochemistry Notes Class 12
electrochemistry electrochemistry class 12 cbse notes pdf download journal free download here cbse class 12 chemistry electrochemistry notes and electro |
|
12th Class Chemistry Notes Cbse All Chapter imgnikoncentercl
1 mar 2021 · Electrochemistry Class 12 Notes Chapter 4 Download Class 12 Chemistry Revision Notes Class 12 Chemistry Practical Notes Pdf |
|
Mcq For Electrochemistry Of Class 12th - Ruforum
answers pdf , electrochemistry class 12 notes chemistry mycbseguide, cbse class 12 mcqs for class 12 chapter wise with answers pdf free download is very |