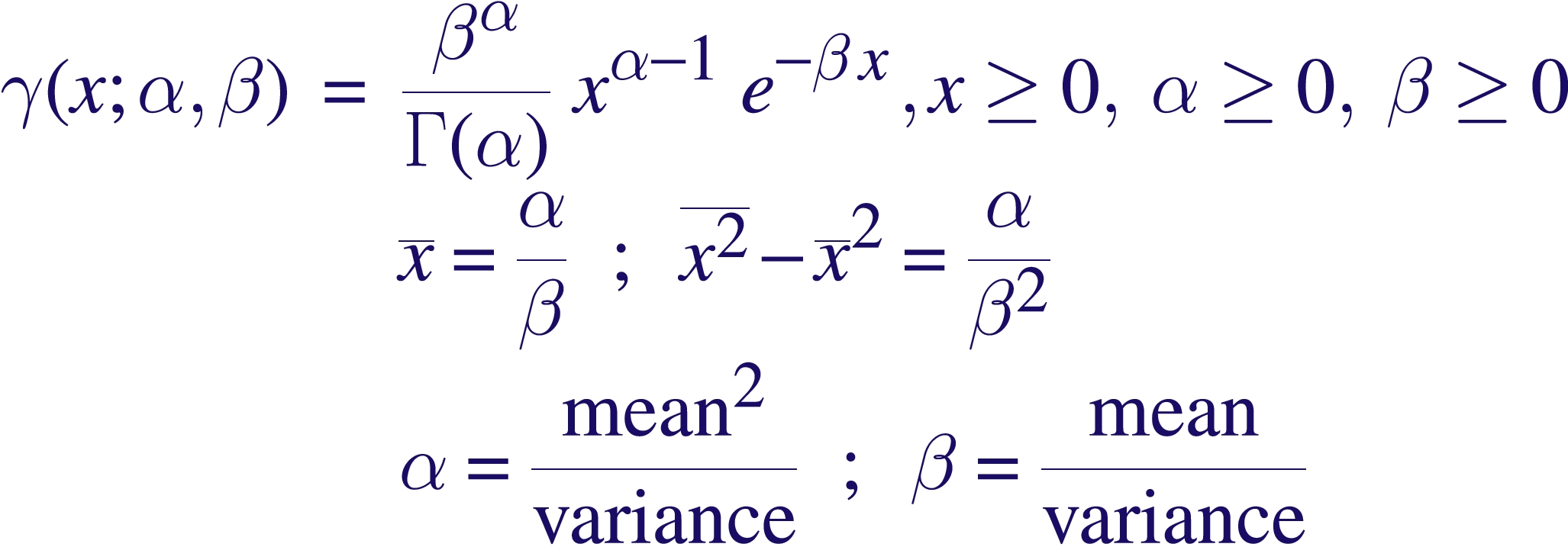cp/cv gamma
|
Measurement of Cp/Cv for Argon Nitrogen
Abstract: The ratio of specific heats γ at constant pressure Cp and constant volume Cv have been determined by measuring the oscillation frequency when a ball bearing undergoes simple harmonic motion due to the gravitational and pressure forces acting upon it |
How do you calculate gamma?
We can define an additional variable called the ratio of specific heats, which is given the Greek symbol “gamma”, which is equal to cp divided by cv: “Gamma” is just a number whose value depends on the state of the gas. For air, gamma = 1.4 for standard day conditions.
What does gamma mean in kinetic theory?
In the kinetic theory of gases, this result is derived from considerations of the conservation of energy at a molecular level. We can define an additional variable called the ratio of specific heats, which is given the Greek symbol “gamma”, which is equal to cp divided by cv: “Gamma” is just a number whose value depends on the state of the gas.
What does CP/CV stand for?
Heat Capacity Ratios for Gases (Cp/Cv) The equipartition theorem states that any quadratic energy term such as kinetic energy contributes equality to the internal energy of a system in thermal equilibrium. This means that for a gas each degree of freedom contributes ½ RT to the internal energy on a molar basis (R is the ideal gas constant)
What is gamma for air?
For air, gamma = 1.4 for standard day conditions. “Gamma” appears in several equations which relate pressure, temperature, and volume during a simple compression or expansion process. Because the value of “gamma” just depends on the state of the gas, there are tables of these values for given gases.
H = E + p * V
where h in the specific enthalpy, p is the pressure, v is the specific volume, and e is the specific internal energy. During a process, the values of these variables will change. Let’s denote the change by the Greek letter delta (which looks like a triangle). So “delta h” means the change of “h” from state 1 to state 2 during a process. Then, for a
Delta H = Delta E + p * Delta V
The enthalpy, internal energy, and volume are all changed, but the pressure remains the same. From our derivation of the enthalpy equation, the change of specific enthalpy is equal to the heat transfer for a constant pressure process: www1.grc.nasa.gov
Delta H = CP * Delta T
where delta T is the change of temperature of the gas during the process, and cis the specific heat capacity. We have added a subscript “p” to the specific heat capacity to remind us that this value only applies to a constant pressure process. The equation of state of a gas relates the temperature, pressure, and volume through a gas constant R . Th
p * Delta V = R * Delta T
Now let us imagine that we have a constant volume processwith our gas that produces exactly the same temperature change as the constant pressure process that we have been discussing. Then the first law thermodynamics tells us: www1.grc.nasa.gov
Delta E = Delta Q – Delta W
where q is the specific heat transfer and wis the work done by the gas. For a constant volume process, the work is equal to zero. And we can express the heat transfer as a constant times the change in temperature. This gives: www1.grc.nasa.gov
Delta E = Cv * Delta T
where delta T is the change of temperature of the gas during the process,and cis the specific heat capacity. We have added a subscript “v” to the specific heat capacity to remind us that this value only applies to a constant volume process. Even though the temperature change is the same for this process and the constant pressure process, the value
CP = Cv + R
The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. This rather remarkable result has been derived from thermodynamic relations, which are based on observations of physical systems and processes. In the kinetic theory of gases, this result is derived from considerations of
Gamma = CP / Cv
“Gamma” is just a number whose value depends on the state of the gas. For air, gamma = 1.4 for standard day conditions. “Gamma” appears in several equations which relate pressure, temperature, and volume during a simple compression or expansion process. Because the value of “gamma” just depends on the state of the gas, there are tables of these val

Cp/Cv=gamma Proof Relation Between Specific Heats & Adiabatic Index Properties of Gases BME

Derivations of KTG 02 Value of Cp Cv and Gamma For Monoatomic Diatomic Polyatomic Gases

Cp/Cv=gammaRatio of two specific heats clear👌 explanation with notes
|
Premier et Second Principes
Pour un gaz diatomique CP = 7. 2. nmolR. On note ? = Cp/Cv il passe de pour l'air ? = 1.4. Pour un gaz parfait on voit que l'on a a. Cp ? CV = nmolR |
|
Determining the Ratio Cp/CV using Ruccharts Method
After measuring the resonant frequency finding gamma involves a simple calculation. THEORY. Before we begin to analyze the mechanics of the Ruchardt apparatus |
|
5. DETERMINAREA RAPORTULUI CALDURILOR MOLARE.pdf
Raportul Cp/Cv = ? se numeste exponent adiabatic fiindca intevine in legea transformarii adiabatice. Se numeste adiabatica o transformare in timpul careia |
|
Gaz et Fluides
On définit le coefficient ? : ? = Cp. Cv. = cp cv. = Cpm. Cvm. 3 Le Gaz Parfait et au-del`a. 3.1 Le gaz parfait. Un gaz parfait est un gaz o`u il n'y a pas |
|
LA THERMODYNAMIQUE
puisque ? = CP /CV = (5/2)(3/2) = 5/3. (b) Lorsque la temp´erature reste constante (T1 = T2) P1V1 = P2V2 conform´ement `a la loi |
|
Transformation adiabatique dun gaz parfait
lorsque l'essence y p´en`etre le m´elange s'enflamme spontan´ement. On trouve que : PV ?. = constante o`u ? est une constante qui vaut. Cp. CV. |
|
Relation between Cp And Cv
Cp & Cv respecti. Q be the. Althoug them to. Step 1: T Cv eral Physics fitted with ure as well a ure and cons nience of calc ... cp – cv =R/M = ? ... |
|
FORMULAIRE PREMIER PRINCIPE Premier principe de la
cP. cV alors CVm = R ? ? 1 et CPm = ?R ? ? 1 . Puisque H et U ne dépendent que de T on peut toujours écrire pour un gaz parfait :. |
|
Termodinámica: Determinación del coeficiente ? (gamma) del aire por
Cp-CV=R y que. R. CV. 2. 3. = y se define el coeficiente. 67.1. 3. 5. ?. = = V p c c ?. Si bien este resultado es muy aproximado para gases monoatómicos |
|
Premier et Second Principes
On note ? = Cp/Cv il passe de pour l'air ? = 1 4 Pour un gaz parfait on voit que l'on a a Cp ? CV = nmolR et CV = nmolR ??1 et CP = ?nmolR ??1 |
|
LA THERMODYNAMIQUE
Le tableau suivant permet de comparer les valeurs th´eoriques donn´ees par la th´eorie cin´etique avec celles exp´erimentales Gaz degr´e de U/mole CV CP ? = |
|
Transformation adiabatique dun gaz parfait
puisque ? = CP /CV = (5/2)(3/2) = 5/3 (b) Lorsque la temp´erature reste constante (T1 = T2) P1V1 = P2V2 conform´ement `a la loi |
|
Formulairepdf
Si on pose ? = CP CV = CPm CVm = cP cV alors CVm = R ? ? 1 et CPm = ?R ? ? 1 Puisque H et U ne dépendent que de T on peut toujours écrire pour un gaz |
|
Chaleur travail et énergie interne des gaz parfaits - AC Nancy Metz
constante : Cv et Cp : la quantité de chaleur Q = M·Cv·?T ou M·Cp ?T développée lors Cp/Cv ? ? or Cp > Cv donc ? > 1 : une adiabatique est plus |
|
Gaz et Fluides - Free
On définit le coefficient ? : ? = Cp Cv = cp cv = Cpm Cvm 3 Le Gaz Parfait et au-del`a 3 1 Le gaz parfait Un gaz parfait est un gaz o`u il n'y a pas |
|
Chapitre 3 LES GAZ PARFAITS : EXEMPLES DE CALCULS DE
R est la constante des gaz parfaits Cv et Cp sont les chaleur spécifiques molaires à volume PV ? = cte avec ? = Cp Cv 3 1 Entropie d'un gaz parfait |
|
Rappels de thermodynamique
Pour un gaz parfait P V = n R T donc CV = CP - R On pose ? = CP / CV Pour un gaz monoatomique on montre que U = 3/2 nRT On tire CV = 3R/2 CP = 5R/2 et |
|
D] Premier principe de la thermodynamique
cv = cp – R => Relation de MAYER On pose ? = (Cp / Cv) qui est une constante • Valeurs de Cp et Cv : Pour un gaz monoatomique : Exemple : H O N |
|
Chapitre III Gaz parfaits
Cp Cv et R exprimées en J/Mole°k adiabatique ? : Coefficient de poisson ou adiabatique avec ici Cp chaleur spécifique à une pression constante |
Comment calculer CP et CV ?
Il s`agit de la quantité de chaleur à fournir à un système pour élever sa température de 1°C. On distingue Cp, capacité calorifique à pression constante et Cv, à volume constant.C'est quoi CP et CV ?
La pression dépend donc de la température : P = nRT /V. Le travail élémentaire reçu est ?PdV = 0. La chaleur reçue est donc égale à la variation d'énergie interne : ?Q = dU = nCvdT ? Q = nCv (T2 ? T1).Comment calculer la quantité de chaleur d'un gaz parfait ?
La capacité calorifique à pression constante, Cp, est égale à la dérivée partielle de l'enthalpie par rapport à la température à pression constante. De même, La capacité calorifique à volume constant, Cv, est égale à la dérivée partielle de l'énergie interne par rapport à la température à volume constant.
| Relation between Cp And Cv - selfstudyin |
| 1 Determining the Ratio C /CV using Rucchart’s Method |
| Measurement of Cp/Cv for Argon Nitrogen |
| Specific Heats: Cv and Cp for Monatomic and Diatomic Gases |
| 1 Determining the Ratio C /CV using Rucchart’s Method - PhysLab |
| Difference in Cp and Cv |
What are the experimental values of gamma?
- Experimental values of ? for air, Helium, and Nitrogen. value std. dev. air 1.31 0.01 Helium 0.46 0.08 Nitrogen 0.62 0.09 The experimental values of gamma for Helium and Nitrogen do not agree with theoretical values.
. For a monatomic gas, such as helium, ?=5/3=1.667.
How is gamma measured?
- In this experiment, this ratio between these two heat capacities, gamma, is measured using an essentially mechanical technique.
. A metal piston, placed between two columns of the same gas, is made to oscillate using a magnetic coil.
What is the gamma value for a diatomic gas?
- This value makes sense theoretically, since the air around us is made up of mostly diatomic gases, and theoretically gamma for a diatomic gas is 1.4.
. Table 2 gives the values for gamma found in this experiment.
. Table 2.
What is the experimental value of gamma for helium and nitrogen?
- The experimental values of gamma for Helium and Nitrogen do not agree with theoretical values.
. For a monatomic gas, such as helium, ?=5/3=1.667.
. The experimental value found in this experiment is not within the error bounds dictated by the standard deviation.
|
Etude de la fonction Gamma Γ
Etude de la fonction Gamma Γ Précis de mathématiques, Analyse MP, page 319 Exercice : On appelle fonction Gamma la fonction définie par Γ : x ↦− → |
|
La fonction Γ - Maths-francefr
2) Relation fonctionnelle Soit x>0 Soient a et A deux réels tels que 0 |
|
Somme de lois gamma differentes et determination des parametres
able `a la distribution Gamma que lorsque toutes les composantes de la somme ont le même param`etre d'échelle Dans le cas général de n lois Γ(αi;βi) ayant |
|
Fonction Gamma dEuler et fonction zêta de Riemann - Département
Pour x > 0 réel, la fonction Gamma est définie par : Γ(x) := ∫ ∞ 0 e−t tx−1 dt Cette intégrale converge près de t = 0, puisque la fonction tx−1 y est intégrable |
|
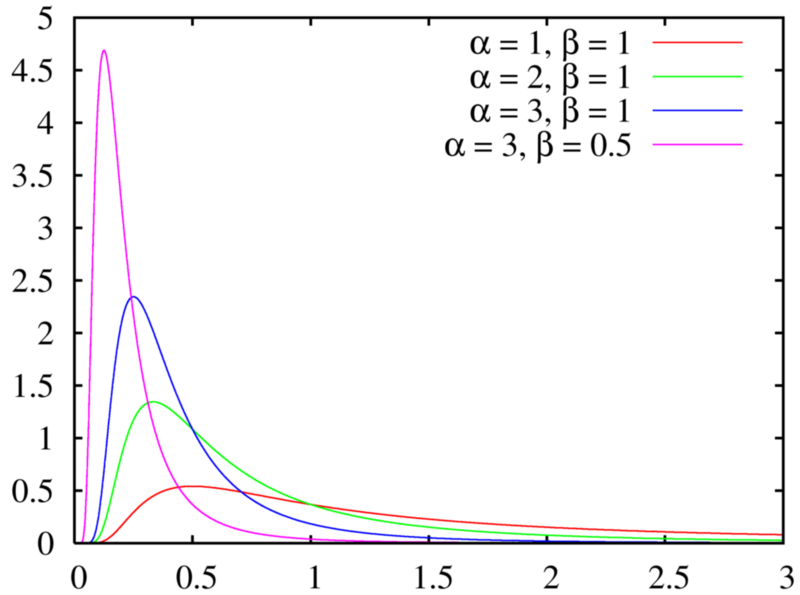
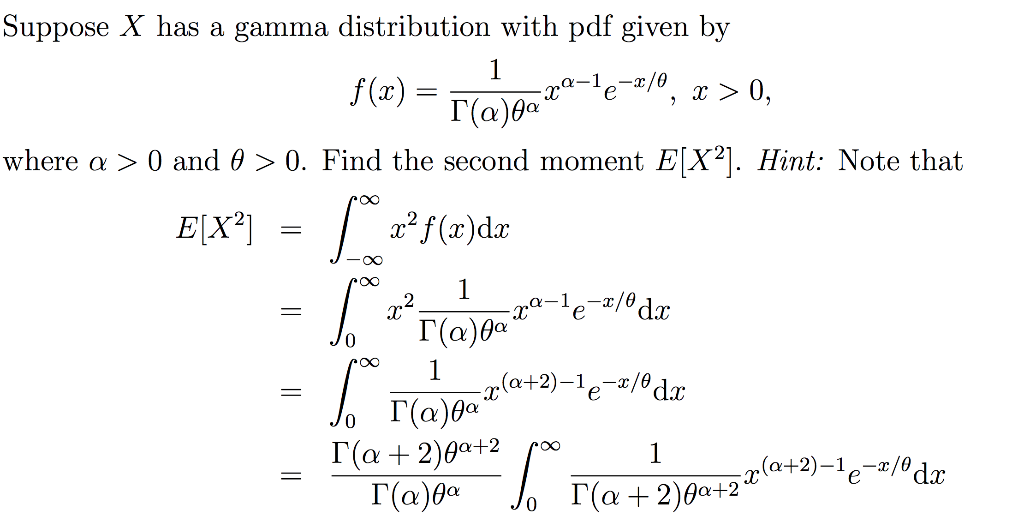
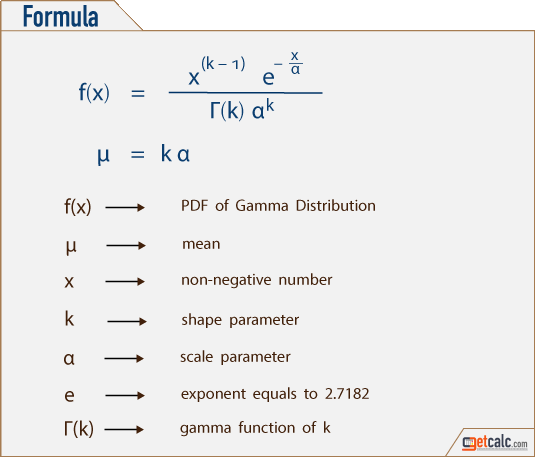
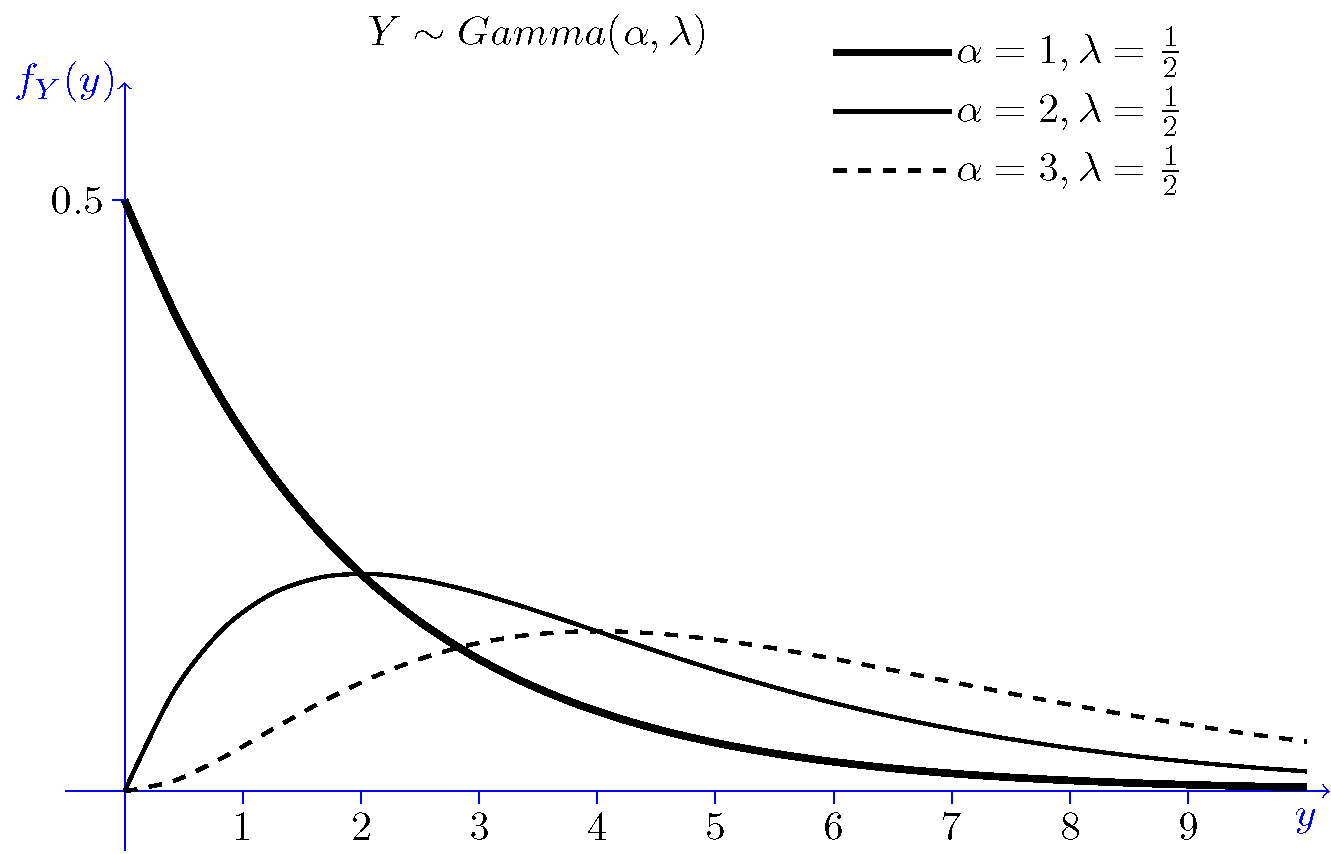
Étude de la loi Gamma
Étude de la loi Gamma Pierron Théo 5 janvier 2014 Soit λ > 0, a > 0 La loi Γ est une loi de probabilité de densité γa,λ : x ↦→ λa Γ(a) e−λxxa−11x⩾0(x) |
|
Gamma-GT FS* - DiaSys Diagnostic Systems GmbH
Gamma-GT FS* Szasz mod /IFCC stand CODE CQN : C1 Réactif de diagnostic in vitro pour la détermination quantitative de gamma-glutamyl-transférase |
|
CHAPITRE 12 Intégrales impropres, fonctions gamma et - LACIM
La fonction gamma apparait dans différents contextes en mathématiques, par exemple en théorie des nombres, en probabilité, etc Nous concluerons ce chapitre |
|
Fermi et lUnivers en rayons gamma - Reflets de la physique
ciel en rayons γ (gamma) depuis l'espace, car ils sont absorbés par l'atmosphère L'instrument principal, le Large Area Telescope ou LAT, est exploité par une |
|
Sur certaines propriétés de la distribution gamma - Numdam
DISTRIBUTIONS GAMMA GENERALISEES Pour rechercher les estimateurs p, â 1 â2 des paramètres p, a 1-' et a2 nous appliquerons la |