grade 12 chemistry textbook answers mcgraw hill
|
Unit-1-sampler-inspire-chemistrypdf
7 jan 2019 · Solve a Problem STEM UNIT PROJECT Optimize Battery Chemistry Investigate a real-world application of chemical reactions: batteries Propose a |
|
McGraw-Hill-Ryerson-Chemistry-11pdf
This chapter will reacquaint you with the science of chemistry You will grade 4 students can understand it 20 Element A with three electrons in |
|
Chemistry-answerspdf
ANSWERS 355 From the chemical equation the number of moles of Fe2O3 is half the number 3 ▷ a i (first answer is shown in the textbook as an example) ii |
|
McGraw-Hill-Ryerson-Chemistry-11pdf
You will learn how to predict the kinds of bonds (the chemical combinations) and the reactions that occur during these interactions Matter and Chemical |
|
McGraw-Hill-Ryerson-High-School-Chemistry-12pdf
What chemical reactions are typical of these compounds? How can you name different organic compounds and represent their structures? What do you need to know in |
|
Grade 12 Chemistry: A Foundation for Implementation
Jan 21 2011 ... McGraw-Hill Higher Education |
|
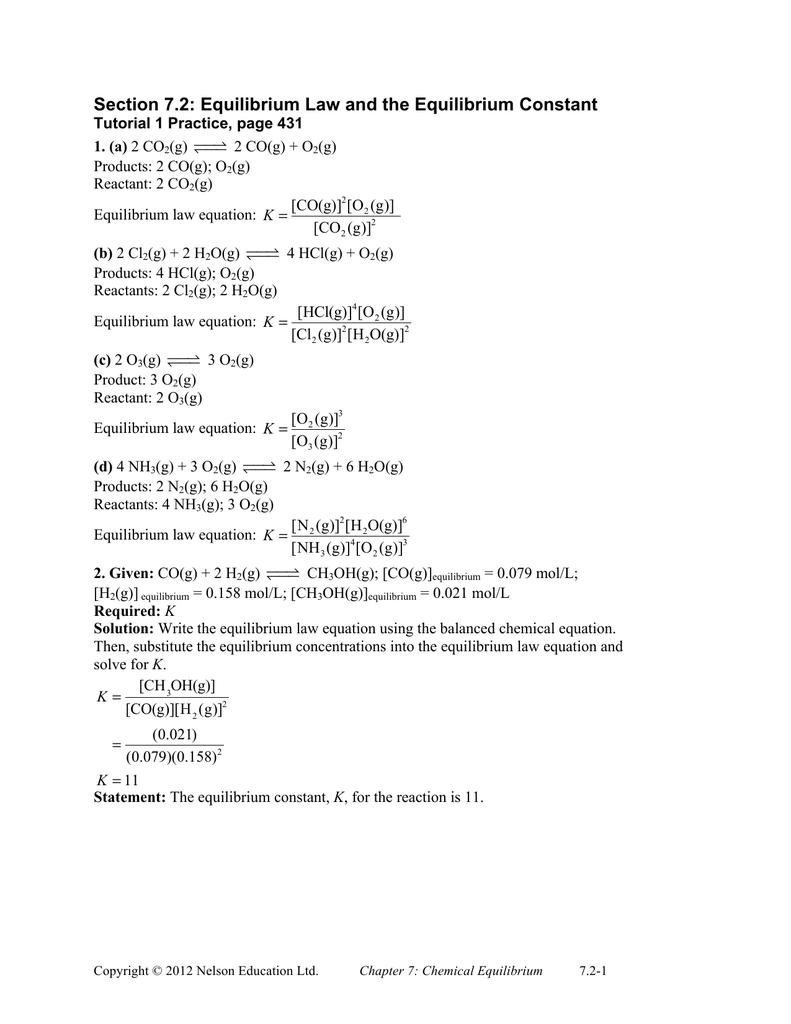
Section 4.3 Chemical Equations Chapter 5 Compounds are
C12H22O11 + 12 O2 → 12 CO2 + 11 H2O. 4. SR. 3 CuSO4 + 2 Fe → Fe2(SO4)3 + 3 Cu. 5. DR. MgF2 + Li2CO3 © 2008 McGraw-Hill Ryerson Limited. MHR • Workbook ... |
|
McGraw-Hill-Ryerson-Chemistry-12.pdf
What about a drink? Milk and juice are solutions of water in which organic compounds are dissolved. In this unit you will study a. |
|
Hebden : Chemistry 12
Some material from my book Chemistry: Theory and Problems Book. Two (McGraw-Hill Ryerson |
|
5.1 - Section - Summary
12. Cr₂O₂ inorganic. 13. CHCI organic. 14. Caco |
|
Unit-1-sampler-inspire-chemistry.pdf
Jan 7 2019 ... McGraw-Hill Education; Tinke Hamming/Ingram Publishing; McGraw-Hill Education ... Do not use the atomic radii values in Figure 12 to answer the ... |
|
BC Science 10 Workbook Answers - Unit 1: Sustaining Earths
12. C. UNIT 2 Chemical Reactions and. Radioactivity. Chapter 4 Atomic theory © 2008 McGraw-Hill Ryerson Limited. Workbook Answers • MHR. 11. Page 12. (b). 3 ... |
|
BC Science 9 Workbook Answers - UNIT 1 Atoms Elements
https://mrsinghscience.files.wordpress.com/2012/10/sc-09-biology-answers.pdf |
|
Untitled
Chemical Equations. © 2008 McGraw-Hill Ryerson Umited. Page 11. 1. Name. Date 12. A solution of sodium sulphide is mixed with a solution of copper(II) nitrate ... |
|
Chem12 SM Ch10 Section10.2 final ok
Analysis: Use the equation !E°r (cell) = E°r (cathode) " E°r (anode) to calculate the standard cell potential. Solution: The half-cell reaction with more |
|
Grade 12 Chemistry: A Foundation for Implementation
Jan 21 2011 promotes the idea that all answers are enshrined in a textbook. The successful implementation of Grade 12 Chemistry depends on a ... |
|
McGraw-Hill-Ryerson-High-School-Chemistry-12.pdf
What about a drink? Milk and juice are solutions of water in which organic compounds are dissolved. In this unit you will study a. |
|
[Chemistry 12 Solutions Manual]
Solutions to Practice Problems in Chapter 1 Structure and Physical. Properties of Organic Compounds. Naming Alkanes. (Student textbook page 19). |
|
Untitled
C2008 McGraw-Hill Ryerson Limited. Acids and Bases • MHA acid base acid. Methyl Rød. Section 5.1. 85. Name. Date. Comprehension. PAR. Use with textbook |
|
Science Notebook - Teacher Edition
McGraw-Hill has developed the Science Notebook know so that they can understand any textbook. ... Organic. Chemistry. I found this information. |
|
Untitled
2008 McGraw-Hill Ryerson Limited www. Seotion 6.3 Organic Compounds MHR 99. Section. Name. Date. 6.1. Types of Chemical Reactions. Summary. Textbook pages |
|
Untitled
2008 McGraw-Hill Ryerson Limited. Assessment only an atom's valence electrons and its chemical symbol. ... to answer question 16. 11p. 12n. |
|
Answers
9. grade 9 41; grade 10 |
|
Chemistry Nelson 12 Textbook Answers - accreditation.ptsem
and install the Chemistry Nelson 12 Textbook Answers it is utterly easy then |
Grade 12 Chemistry
Explore advanced concepts and applications in chemistry tailored for Grade 12 students.
Examples with Explanations
Gain clarity on complex topics through examples:
- Example 1: Demonstrating the concept of stoichiometry by calculating the amount of product formed in a reaction. For instance, in the reaction 2H₂ + O₂ → 2H₂O, if 4 moles of hydrogen gas react, the stoichiometry dictates that 2 moles of water will be produced.
- Example 2: Explaining the behavior of gases using the ideal gas law equation PV = nRT. Consider a scenario where a gas occupies a volume of 5 liters at a pressure of 2 atmospheres and a temperature of 300 Kelvin. Using the ideal gas law, you can calculate the number of moles of gas present.
- Example 3: Illustrating acid-base titration by determining the concentration of an unknown acid solution. By carefully adding a standardized base solution to the acid solution and monitoring the pH change, the equivalence point can be determined, allowing for the calculation of the acid concentration.
Practice Exercises
Enhance your understanding with these practice problems:
- Calculate the pH of a solution with a hydrogen ion concentration of 1 x 10^-3 M.
Correct Answer: pH = 3 - Identify the oxidation state of manganese in KMnO₄.
Correct Answer: +7 - Calculate the heat released in a reaction if 50 grams of methane (CH₄) reacts with excess oxygen to produce carbon dioxide and water, given that the enthalpy change for the reaction is -890 kJ/mol.
Correct Answer: -222.5 kJ
Case Studies
Explore real-world applications and implications of chemistry:
- Case Study 1: Analyzing the process of polymerization in the production of plastics. Understand the role of catalysts, monomers, and reaction conditions in controlling the properties of the resulting polymer.
- Case Study 2: Investigating the use of chromatography in forensic science. Learn how chromatographic techniques can separate and analyze complex mixtures, aiding in the identification of substances at crime scenes.
- Case Study 3: Examining the environmental impact of acid rain on ecosystems. Explore the chemical reactions involved in the formation of acid rain and their effects on soil, water, and living organisms.
Important Notes
- Chemistry involves the study of matter, its properties, composition, and interactions.
- Understanding chemical bonding is crucial for predicting the behavior of substances in reactions.
- Thermodynamics and kinetics govern the energy changes and rates of chemical reactions, respectively.
- Environmental chemistry examines the impact of human activities on the environment and strategies for mitigation.
By grasping these key concepts, students can excel in Grade 12 chemistry and beyond.
Subcategories
Dive deeper into specialized areas of chemistry:
- Organic chemistry: Explore the structure, properties, and reactions of carbon-containing compounds, including hydrocarbons, alcohols, and carboxylic acids.
- Inorganic chemistry: Investigate the properties and behavior of inorganic substances, such as metals, non-metals, and metalloids, and their compounds.
- Physical chemistry: Delve into the principles governing the behavior of matter and energy in chemical systems, including thermodynamics, kinetics, and quantum mechanics.
- Analytical chemistry: Learn techniques for qualitative and quantitative analysis of substances, including spectroscopy, chromatography, and electrochemistry.
Exploring these subcategories provides a comprehensive understanding of the diverse branches of chemistry.
Step-by-Step Guide
Follow these steps to excel in Grade 12 chemistry:
- Review fundamental concepts from earlier grades, including atomic structure, chemical bonding, and stoichiometry.
- Engage actively in classroom activities, laboratory experiments, and discussions to reinforce learning.
- Practice solving a variety of problems, ranging from basic calculations to complex applications of concepts.
- Seek additional resources such as textbooks, online tutorials, and study groups to clarify doubts and deepen understanding.
- Stay updated with current developments in the field of chemistry through scientific journals, news articles, and documentaries.
FAQs
- Q: How can I improve my understanding of chemical reactions?
A: Practice balancing chemical equations and analyzing reaction mechanisms to develop proficiency. - Q: What careers can I pursue with a background in chemistry?
A: Chemistry opens doors to various career paths, including research, medicine, environmental science, and chemical engineering. - Q: What is the significance of laboratory experiments in chemistry?
A: Laboratory experiments provide hands-on experience and reinforce theoretical concepts, fostering critical thinking and problem-solving skills.
Multiple Choice Questions
- What is the hybridization of the carbon atom in methane (CH₄)?
Correct Answer: sp³ - Which of the following is a strong acid?
Correct Answer: HCl - What is the formula of sodium chloride?
Correct Answer: NaCl
About the Topic
Grade 12 chemistry builds upon foundational concepts from earlier grades, providing a deeper understanding of the principles governing matter and its transformations. Mastery of these concepts is essential for success in further studies and careers in science and technology.
Key Elements to Remember
1. Understanding fundamental concepts is crucial for success in Grade 12 chemistry.
2. Practice problem-solving regularly to reinforce learning and improve proficiency.
3. Stay curious and explore the diverse applications of chemistry in everyday life and beyond.
4. Seek assistance from teachers, peers, and online resources to clarify doubts and deepen understanding.
|
Answers To Mcgraw Hill Chemistry Grade 12 - Driven With Skip Barber
Chemistry Grade 12 Textbook Answers GradeSaver Mcgraw Hill Chemistry 12 Answers connect mcgraw- hill com answers? Yahoo Answers Mcgraw Hill |
|
Grade 12 Nelson Chemistry Textbook Answers - 50000+ Free
Answers Yeah, reviewing a ebook grade 12 nelson chemistry textbook Nelson Chemistry Grade 12 Textbook - The student edition of this McGraw-Hill |
|
From McGraw-Hill Ryerson - McGraw Hill Canada
e McGraw-Hill Ryerson's Chemistry 12? Abundance of connecTschool™ access complements the textbook and is conveniently available anywhere, All Answers in one easy-to-find location chapter 2 reactions of organic compounds 2 1 |
|
Mcgraw Hill Chemistry 12 Solutions Manual - DITP
We manage to pay for mcgraw hill chemistry 12 solutions manual and numerous ebook Unit 2 - solutions to McGraw-Hill grade 12 textbook - StuDocu |
|
Chemistry 12 Mcgraw Hill Ryerson Solutions Manual
mcgraw hill ryerson chemistry 12 solution manual 60 mcgraw hill ryerson number of organic compounds with reference to the unique nature of of the 12 mcgraw hill ryerson 2011 textbook mcgraw or type in mcgrawhill chemistry 11 online |
|
Answers To Mcgraw Hill Chemistry Grade 12
Answers To Mcgraw Hill Chemistry Grade 12 When people should go to the book stores, search introduction by shop, shelf Textbook Answers GradeSaver |