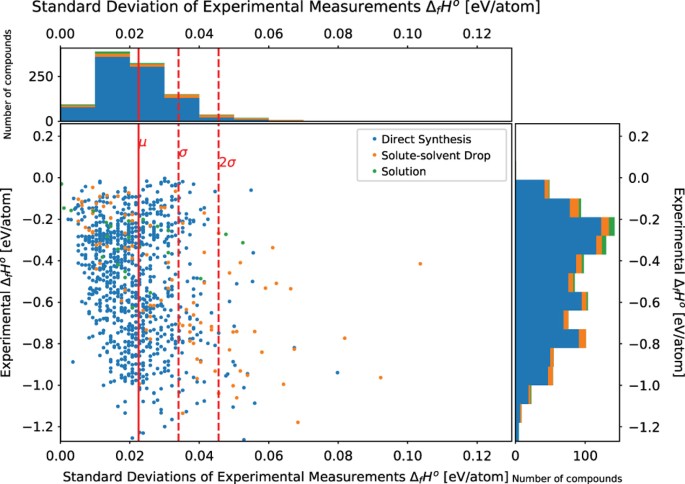
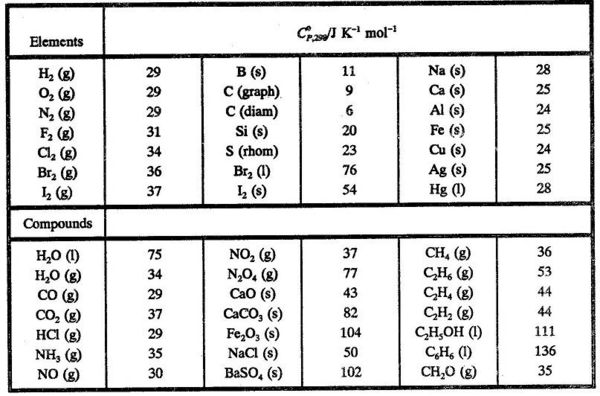
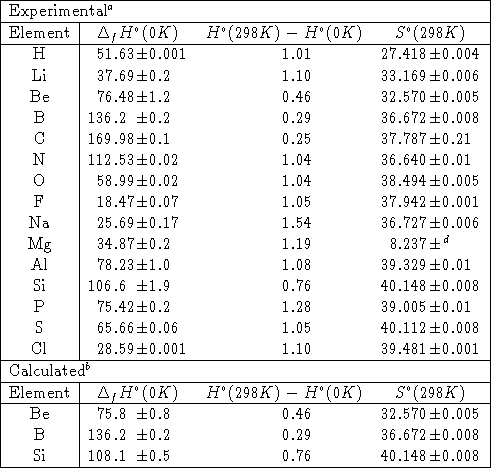
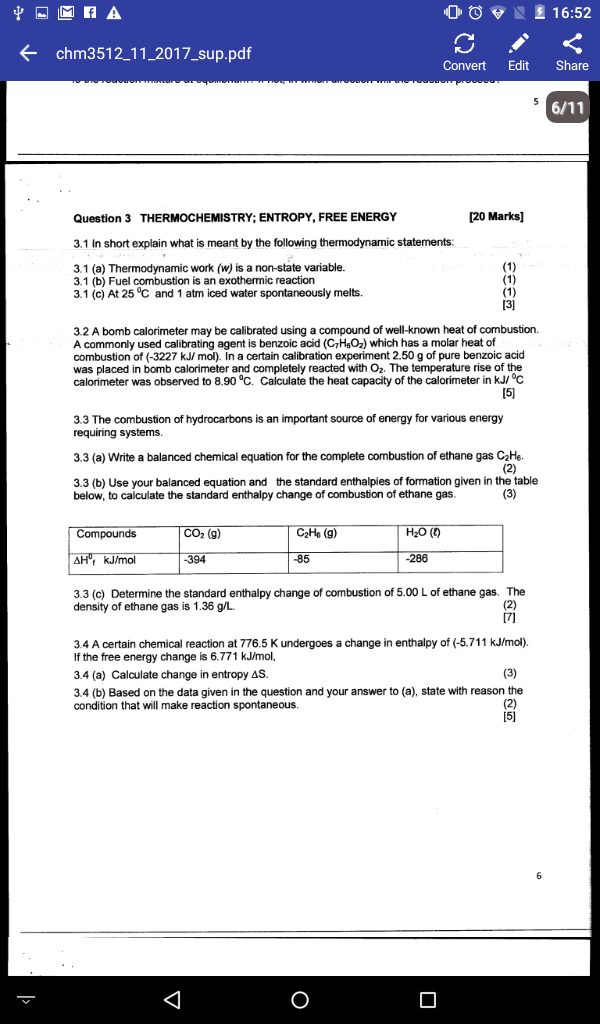
enthalpies standard de formation et entropie standard
|
Standand Enthalpies of Formation & Standard Entropies of
Standand Enthalpies of Formation & Standard Entropies of Common Compounds Substance State ∆H f S (kJmol) (Jmol·K) Ag s 0 42 6 Ag+ aq 105 79 72 7 AgCl s −127 01 96 2 |
|
Standard Enthalpies of Formation
Standard Enthalpies of Formation Alan D Earhart 11/7/2016 Substance ΔH° f (kJ/mol) Substance ΔH° f (kJ/mol) Substance ΔH° f (kJ/mol) AgCl(s) -127 0 CaSO 4(s) -1434 5 N 2H 4(g) +95 4 Al 2O 3(s)-1675 7 Fe 2O 3(s) -824 2 N 2H 4(l) +50 6 CHCl 3(g) -103 2 HBr(g) -36 4 N 2O(g) +82 1 CH 2Cl 2(g) -95 5 HCl(g) -92 3 N 2O 4(g) +9 1 CH |
|
Standard Enthalpy of Formation* for Various Compounds
Standard Enthalpy of Formation* for Various Compounds Compound ΔH˚ f (kJ/mol) Compound ΔH˚ f (kJ/mol) Compound ΔH˚ f (kJ/mol) Compound ΔH˚ f (kJ/mol) Ag 2O(s) −30 6 C 2H 5OH(l) −277 6 HCl(g) −92 3 NH 4Cl(s) −315 4 Ag 2S(s) −31 8 C 2H 6(g) −84 7 HF(g) −268 6 NH 4NO 3(s) −365 1 AgBr(s) −99 5 C 3H |
Does graphite have a standard enthalpy of formation equal to zero?
To determine which form is zero, the more stable form of carbon is chosen. This is also the form with the lowest enthalpy, so graphite has a standard enthalpy of formation equal to zero. Table 1 provides sample values of standard enthalpies of formation of various compounds. Table 1: Sample Table of Standard Enthalpy of Formation Values.
What does O F mean in enthalpy?
o = A degree signifies that it's a standard enthalpy change. f = The f indicates that the substance is formed from its elements The equation for the standard enthalpy change of formation (originating from Enthalpy's being a State Function ), shown below, is commonly used:
What is standard enthalpy of formation?
standard enthalpy of formation (ΔHchange in enthalpy that accompanies the formation of 1 mole of a compound from its elements with all substances in their standard states The table below shows the standard enthalpy of formationstandard Gibbs free energy of formation, at constant pressure of several inorganic compounds.
What is the enthalpy of phosphorus?
The reference form in phosphorus is not the most stable form, red phosphorus, but the less stable form, white phosphorus. Recall that standard enthalpies of formation can be either positive or negative. The enthalpy of formation of carbon dioxide at 298.15K is ΔH f = -393.5 kJ/mol CO 2 (g).
|
Les dosages acido-basiques
mol-1) enthalpie libre de formation standard |
|
4. Thermochimie
linéaire des enthalpies standard de formation de ses réactifs et produits: Les valeurs des enthalpie et entropie standard ΔH0r et ΔS0r |
|
Exemples denthalpies standard
dS = CpdT/T. Par intégration : S (T1) – S(T2) = Cp LnT2/T1 si Cp indépendant de T. II. Le troisième principe de la thermodynamique ; entropie molaire standard d |
|
UNIVERSITE PIERRE ET MARIE CURIE
11 juin 2003 Dans le tableau suivant sont rassemblées les valeurs des enthalpies standard de formation ... entropie standard ∆S°(T) en fonction de T ( on. |
|
CHM-1903 Chimie des eaux - Recueil de données
25 août 2015 Enthalpie de formation standard. Enthalpie libre de formation standard. Entropie standard. Capacité calorifique standard o f. H. ∆. (en kJ.mol— ... |
|
Solutions de la série N°3 (exercice 34 et 5) (Thermodynamique)
1- Quelle est l'enthalpie standard de formation de l'octane gazeux à 298 K. 1- Calculer la variation d'entropie standard à 25°C accompagnant les réactions de ... |
|
«EXERCICES ET PROBLEMES CORRIGES DE
l'entropie molaire standard de formation l'entropie molaire standard absolue |
|
Sans titre
coîncide avec le signe positif de l'entropie standard de formation de la réaction. molaire standard de formation et l'enthalpie molaire standard de formation ... |
|
TD chimie n°3 - Calcul des grandeurs de réaction 1 Réactions
ainsi que les enthalpies standard de formations Déterminer l'enthalpie standard ∆rH0 l'entropie standard ∆rS0 et l'enthalpie libre standard ∆rG0 de. |
|
Affinité chimique_exercices_
libre standard de formation : 1934 kJ. mol et entropie standard du diiode gazeux : 260 J. K . mol . 1. Calculer l'enthalpie standard de sublimation |
|
Exemples denthalpies standard
Notée ?fHo ; elle correspond à l'enthalpie standard de formation d'un corps composé par la réaction de formation de ce corps à partir des éléments pris |
|
Deuxième principe et entropie - Exemples denthalpies standard
La variation de l'entropie lors d'une transformation d'un état 1 vers un état 2 Le troisième principe de la thermodynamique ; entropie molaire standard |
|
Zzz_suppexos_th7_thermochimiepdf
écrire l'équation de sa combustion dans le dioxygène de l'air 2 Calculer son enthalpie standard de formation à 298K sachant |
|
4 Thermochimie - EPFL
pure on parlera d'une enthalpie standard de vaporisation notée ?H0vap Exemple : L'enthalpie standard de formation de l'éthanol est obtenue à partir |
|
Thermochimie/equilibres chimiques - Faculté des sciences de meknès
On peut calculer l'enthalpie standard de la réaction à partir des enthalpies standards molaires de formation Loi de HESS : La variation de l'enthalpie standard |
|
Détermination de lenthalpie standard de formation de lion
function is the standard enthalpy of formation of the tetrahydroaluminate (III) ion (Al(OH)4 ques (enthalpie libre enthalpie et entropie de formation) |
|
CHM-1903 Chimie des eaux - Recueil de données
25 août 2015 · Enthalpie de formation standard Enthalpie libre de formation standard Entropie standard Capacité calorifique standard |
|
Thermodynamique - Faculté de Médecine dOran
Q6- On donne à 298 K l'enthalpie standard de combustion du phénol C6H6O et les enthalpies standards de formation de CO2 et de H2O |
|
Solutions de la série N°3 (exercice 34 et 5) (Thermodynamique)
Calculer l'enthalpie standard ?H°r298K de la réaction suivante : CO (g)+ 3H2(g) ? CH4(g) + H2O (l) a) En déduire la valeur de l'énergie interne ?U°r298K |
|
PREMIER PRINCIPE DE LA THERMODYNAMIQUE ENERGIE
Le volume molaire d'un gaz à l'état standard et pour T = 29815K est donc : L'enthalpie de formation des corps simples dans leur état standard de |
|
Exemples denthalpies standard
Enthalpie libre standard de réaction : Notée r 0 Δ G ; elle peut être calculée de plusieurs façons : • soit à partir des f 0 Δ G enthalpie libre de formation données |
|
Reaction chimique - Thermodynamique - Cinétique
chaleurs de combustion, de dissolution ou de changement d'états, il est Notée ∆fHo ; elle correspond à l'enthalpie standard de formation d'un corps composé |
|
PREMIER PRINCIPE DE LA THERMODYNAMIQUE ENERGIE
L'enthalpie standard d'une réaction est la combinaison linéaire des enthalpies standards des réactions qui la constituent Exemple Déterminer de l'enthalpie |
|
Les grandeurs standard - UNF3S
Lorsque tous les constituants du système sont dans leur état standard ∆ r H → ∆ r H0 : enthalpie standard de réaction ∆ r S → ∆ r S0 : entropie standard |
|
4 Thermochimie - EPFL
L'enthalpie standard de réaction par mole d'éthanol formée est ΔH0f (C2H5OH, l ) = – 555 38 kJ / 2 = – 277 69 kJ⋅mol–1 L'enthalpie standard de toute réaction |
|
Doc cours : grandeurs standards de réaction
où Hi°: enthalpie molaire standard * énergie interne standard de réaction ∆rU° : ∆rU° = ∑ νi*Ui° où Ui °: énergie interne molaire standard * entropie standard |
|
Enthalpie de Formation Enthalpie de Formation Supplément
1 Calculer l'enthalpie standard de formation de l'eau à l'état gazeux ∆fH0(H2O( g)) 2 |
|
Thermodynamique de la transformation chimique - Frédéric Legrand
Pour calculer l'enthalpie standard d'une réaction chimique, il suffit de combiner les enthalpies de formation des réactifs et des produits de la manière suivante : |
|
Faculté des Sciences Meknès Cours THERMOCIMIE-EQUILIBRES
On peut calculer l'enthalpie standard de la réaction à partir des enthalpies standards molaires de formation Loi de HESS : La variation de l'enthalpie standard d' |


![PDF] Group additivity values for enthalpies of formation (298 K PDF] Group additivity values for enthalpies of formation (298 K](https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/90524d0fd895f9ca6dacc05b82804182/thumb_1200_1697.png)