claforan dilution
|
ANTIBIOTIQUES UTILISATION PRATIQUE DES ANTI-INFECTIEUX
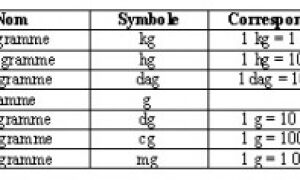
Dilution Stabilité 1g/200mg 1g • Utilisation immédiate 24MUI • IM profonde CLAFORAN • Eau PPI • IM : 4mL (250mg/mL) • IV : 4-10mL (100-250mg/mL) • |
|
CEFOTAXIME PANPHARMA 05g-1g
27 jui 2001 · Adultes : - 3g/jour en moyenne pouvant être portée jusqu'à 12g selon la sévérité de l'infection - dans les infections urinaires |
|
Claforan (céfotaxime)
12 nov 2014 · Pour la perfusion intraveineuse La solution reconstituée peut ensuite être diluée dans 50 à 1000 mL d'un soluté recommandé pour perfusion |
|
Claforan® IV Injection 05 g Claforan® IV Injection 10 g Claforan
Claforan is prepared according to the WFI dilution table and shaken well until the powder has dissolved High concentrations of cefotaxime and desacetyl- |
|
CLAFORAN®
050547s066lbl.pdf |
|
GUIDE DADMINISTRATION DES
Dilution avec NaCl 0 9 ou G5 (conc : 0 5 à 2 5 mg/mL) Dilution standard perfusion IV continue : PSE : 100 mg = 50 mL (conc : 2 mg/mL) Stable 24 h |
|
GUIDE DADMINISTRATION DES
pdf Ne pas diluer IV lent (Débit max 4 mL/min) USI-NEONAT : Non filtrable sur filtre en ligne PALL NEO96 ou ELD96 0 2 microns ou NLF2E ou TNA1 1 2 microns |
|
GUIDE de reconstitution et dutilisation des MÉDICAMENTS
Céfotaxime ---> voir CLAFORAN Ceftazidime ---> voir FORTUM Ceftriaxone Dilution dans 500ml de NaCl 09 ou G 5 (1ml = 02mg) Conservation des ampoules |
Comment diluer le Claforan ?
Diluer Claforan 1000 mg dans 4 ml d'eau pour préparations injectables.
Comment diluer un médicament ?
Remplissez le gobelet d'eau jusqu'au trait de graduation.
Débouchez le flacon et versez-y les deux tiers de l'eau.
Puis refermez bien la bouteille et agitez-la durant une minute, en la retournant régulièrement.Comment diluer la céfotaxime ?
En cas d'injection intramusculaire, dissoudre CEFOTAXIME VIATRIS 1 g, poudre pour solution injectable (IM - IV) dans 4 ml d'eau pour préparations injectables.
- Diluer sur la base de 1 g de poudre d'amoxicilline dans 20 ml d'eau pour préparations injectables.
|
GUIDE DADMINISTRATION DES ANTI-INFECTIEUX INJECTABLES
Dilution et administration. Conservation après dilution. Remarques. Cefotaxime. (Claforan®). 1g. Reconstitution : +10ml EPPI si IV et IVD pour 1g. |
|
ANTIBIOTIQUES UTILISATION PRATIQUE DES ANTI-INFECTIEUX
Dilution. Stabilité. 1g/200mg. 1g. • Utilisation immédiate CLAFORAN. • Eau PPI. • IM : 4mL (250mg/mL). • IV : 4-10mL (100-250mg/mL). • NaCl09% ou G5%. |
|
NOTICE : INFORMATION DE LUTILISATEUR Claforan 1000 mg
Claforan 1000 mg poudre et solvant pour solution injectable céfotaxime Diluer Claforan 1000 mg dans 4 ml d'eau pour préparations injectables. |
|
GUIDE DADMINISTRATION DES MÉDICAMENTS INJECTABLES
Respecter la chronologie des phases de reconstitution puis de dilution. • Respecter les infos pour le solvant de bicarbonate de sodium cefotaxime. |
|
Claforan.pdf
12 nov. 2014 CLAFORAN (céfotaxime pour injection) est un antibiotique ... ambiante (15 à 25 °C) alors que la dilution a été faite dans des conditions. |
|
TDCalculsdoses pediatriquesCORRIGES
CLAFORAN 40 mg x 4 en IVL sur 15 minutes. (Flacon de 0.5 g de produit actif en poudre à diluer dans 5 ml) Comment effectuez vous la dilution ? |
|
Les dilutions de médicaments
Les dilutions de médicaments. Version : 2 Dilution. Voie et temps de perfusion. Acupan. (Ampoule de 20mg/2ml) ... Claforan ou Céfotaxime (Flacon. |
|
OPC EN300003 Tableau de stabilité des injectables
reconstitution et dilution: utilisation immédiate. Céfotaxime IV IM. Claforan dilution perfusion immédiate à conserver à l'abri de la lumière. |
|
Titre de votre présentation
12 juin 2018 Volume de dilution limité ( 50 cc). Encombrant. 3%. Pompe volumétrique ... céfazoline céfotaxime ... de dilution (Nacl ? G5 ? eau PPI). |
|
Preparing and administering injectable antibiotics: How to avoid
the reconstitution/dilution stage the user is solely responsi- ticarcillin |
|
CLAFORAN Sterile (cefotaxime for injection USP) and
050596s042lbl.pdf |
|
CLAFORAN Sterile (cefotaxime for injection USP) and
050547s066lbl.pdf |
|
PRODUCT MONOGRAPH - Sanofi
CLAFORAN (cefotaxime for injection) is a semi-synthetic 2-aminothiazolyl cephalosporin antibiotic for parenteral use In vitro studies indicate that the antibacterial action of CLAFORAN results from inhibition of cell wall synthesis It is stable against the action of most ?-lactamases INDICATIONS AND CLINICAL USE Treatment |
|
Searches related to claforan dilution PDF
Dilution Table: Claforan may be administered by intravenous infusion 1-2g are dissolved in 40-100ml of Water for Injection or in the infusion fluids listed under "Pharmaceutical Particulars" in Section 6 6 Instructions for use/handling The prepared infusion may be administered over 20-60 minutes |
What is the diluent for Claforan sterile?
CLAFORAN Sterile 1 g or 2 g may be reconstituted in 50 mL or 100 mL of 5% Dextrose or 0.9% Sodium Chloride in the ADD-Vantage diluent container. Refer to enclosed, separate INSTRUCTIONS FOR ADD-VANTAGE SYSTEM. Compatibility and Stability Solutions of CLAFORAN Sterile reconstituted as described above (Preparation of CLAFORAN Sterile)
What are the dosage guidelines for Claforan?
GUIDELINES FOR DOSAGE OF CLAFORAN. Infants and Children (1 month to 12 years): For body weights less than 50 kg, the recommended daily dose is 50 to 180 mg/kg IM or IV body weight divided into four to six equal doses. The higher dosages should be used for more severe or serious infections, including meningitis.
What is the CAS number for Claforan?
CLAFORAN contains approximately 50.5 mg (2.2 mEq) of sodium per gram of cefotaxime activity. Solutions of CLAFORAN range from very pale yellow to light amber depending on the concentration and the diluent used. The pH of the injectable solutions usually ranges from 5.0 to 7.5. The CAS Registry Number is 64485-93-4. S N C N OCH3 CONH O H2N N S COONa
What is Claforan used for?
Treatment CLAFORAN is indicated for the treatment of patients with serious infections caused by susceptible strains of the designated microorganisms in the diseases listed below. (1) Lower respiratory tract infections,including pneumonia, caused by Streptococcus pneumoniae
|
CLAFORAN Sterile (cefotaxime for injection USP) and
050596s042lbl.pdf |
|
CLAFORAN Sterile (cefotaxime for injection USP) and
050547s066lbl.pdf |
|
CLAFORAN Sterile (cefotaxime for injection USP) and
050596s037lbl.pdf |
|
CLAFORAN Sterile (cefotaxime for injection USP) and
050596s040lbl.pdf |
| PRODUCT MONOGRAPH - Sanofi |
| Searches related to claforan dilution filetype:pdf |
|
Administration IV des anti-infectieux - Centre Hospitalier de Sambre
Dilution et administration Conservation Dilution : 250mg qsp 50ml SSI (maxi 5mg/ml) ac clav, céfépime, ceftriaxone, céfuroxime, cefotaxime, cloxacilline, |
|
Guide de reconstitution et dadministration des - OMéDIT Centre
Cefotaxime OUI OUI dilution : 24 h à t°C < 25°C Jamais par IVD ; Perfusion IV lente en 2 h minimum IVD lente 3 à 5 min : diluer la solution primaire de |
|
ANTIBIOTIQUES UTILISATION PRATIQUE DES ANTI-INFECTIEUX
Nom de la spécialité Reconstitution Stabilité Dilution Stabilité Amikacine AMIKLIN CLAFORAN • Eau PPI Dose de 500mg : diluer 5,7 mL de la solution |
|
Antibiotiques - Edimark
dilution pour • Érythème maculo- perfusion Céfotaxime • Méningites à : Listeria Flacon i v (poudre) 70-100 mg/kg/j i v d dilué dans eau Insuffisance Idem |
|
GUIDE de reconstitution et dutilisation des MÉDICAMENTS
Perfusion : Dilution à raison de 500 mg dans 200 ml de NaCl 0,9 ou G 5 CLAFORAN 0,5g et 1g ; pour IM : chl de lidocaïne = Solvants de reconstitution |
|
ANTIBIOTHÉRAPIE CURATIVE-2019 - GILAR
crire de Ceftriaxone ou de Céfotaxime en 1ère intention Pas d'association Le nombre de perfusions dépend de la stabilité de la dilution PERFUSION |
|
TDCalculsdoses pediatriquesCORRIGES - CH Carcassonne
CLAFORAN 40 mg x 4 en IVL sur 15 minutes (Flacon de 500 mg de produit actif en poudre à diluer dans 5 ml) Comment effectuez vous la dilution ? |