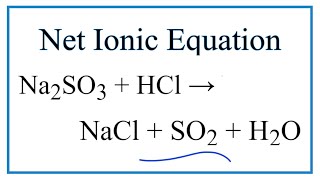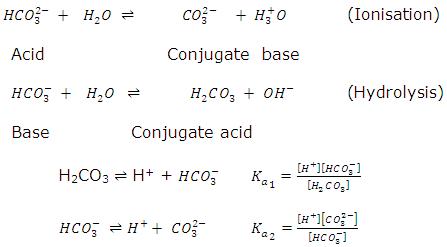na2co3 hydrolysis net ionic equation
|
REACTIONS OF SALTS WITH WATER
Setting the K-expression in the usual way we find for NaC2H3O2 where - - C2H3O2 (aq) + H2O (l) HC2H3O2 (aq) + OH (aq) Kb= [HC2H3O2] [OH -] - [C2H2O2 ] Values for Kb cannot be found in tables Instead they can be derived from values of other equilibrium constants that are found in tables |
How do you write a net ionic equation for a reaction?
This is the net ionic equation for the reaction. Write a net ionic equation to describe the reaction that occurs when 0.100 M K 3 PO 4 solution is mixed with 0.100 M Ca (NO 3) 2 solution Step 2: There are two possible combinations of ions here: K + + NO 3- (forming KNO 3) and Ca 2+ + PO 43- (forming Ca 3 (PO 4) 2 ).
How do you calculate % hydrolysis of Na2CO3?
Write the net ionic equation for the hydrolysis of Na2CO3. Write the Kb expression for the hydrolysis reaction. Calculate the value of Kb for this salt from tabulated values of equilibrium constants. ( Ka2 for HCO3 = 4.7 x 10-11) - 4. From the above Kb find the theoretical [OH], then calculate the theoretical % hydrolysis. x 0.10- x
Is Na2CO3 a chemical reaction?
The (aq) shows that they are aqueous – dissolved in water. The equation for Na2CO3 (Sodium carbonate) and H2O sometimes isn’t considered a chemical reaction since it is easy to change the Na+ and CO3 2- back to Na2CO3 (just let the H2O evaporate). At the same time, the Na2CO3 is a very different substance than Na+ and CO3 2-.
What are the net ionic equations for hydrolysis?
What are the net ionic equations for the hydrolysis of the the following: 1) C2H3O2- + H2O <-----> C2H3O2H + OH-Kb = [HC2H3O2] [OH-] / [C2H3O2-] 2)Carbonate ion is derived from carbonic acid, the acid obtained when carbon dioxide gas is dissolved in water. This acid has two acidic protons, so that means there are two hydro …
|
Exp 17 REACTIONS OF SALTS WITH WATER F 08
Hydrolysis as applied to water solutions of inorganic compounds can be defined as the Write the net ionic equation for the hydrolysis of Na2CO3. |
|
Hydrolysis: Examples:
spectators are eliminatedin net ionic equations (NIE's) for hydrolysis! ions. Eg.) Determine whether the salt sodium carbonate (Na2CO3) is acidic ... |
|
Sec 4.13 – Hydrolysis (notes)
spectators are eliminated in net ionic equations for hydrolysis! Eg.) Determine whether the salt sodium carbonate (Na2CO3) is acidic basic or neutral ... |
|
Untitled
salts both anion and cation hydrolyze and the resulting pH depends on which to write a net ionic equation for each hydrolysis. |
|
EXPERIMENT 11
b) Write net ionic equations representing the hydrolysis of salts Na2CO3. 10-11. Sodium hydrogen carbonate. NaHCO3. 7-8. Sodium phosphate. |
|
ACID/BASE REVIEW
A. Na2CO3(s) + H2O(l) ? Na2O(aq) + CO2(g) + H2O(l) C. Na2CO3(s) ? 2Na+ ... The net ionic equation for the hydrolysis that occurs when KF is dissolved ... |
|
91317 Hydrolysis of Salts
Salts on the other hand |
|
911 Metallurgist
spectators are eliminated in net ionic equations (NIE's) for hydrolysis! ions. Eg.) Determine whether the salt sodium carbonate (Na2CO3) is acidic ... |
|
Untitled
The net ionic equation for the hydrolysis of Na2CO3 is. A. H2O + Na NaOH + H* The balanced formula equation for the neutralization of H2SO4 by KOH is. |
|
Reactions of Salt with Water
reaction of water with one or both ions of a salt to form a weak acid and a OH- or a weak base and Write the net ionic equation for the hydrolysis of Na2CO3 2 |
|
Hydrolysis - SSS Chemistry - D Colgur
spectators are eliminatedin net ionic equations (NIE's) for hydrolysis Eg ) Determine whether the salt sodium carbonate (Na2CO3) is acidic, basic or neutral in |
|
Hydrolysis - Arcuric Acid
spectators are eliminated in net ionic equations for hydrolysis Eg ) Determine whether the salt sodium carbonate (Na2CO3) is acidic, basic or neutral in |
|
1) What is the net ionic equation for the hydrolysis of NH 4ClO4? (1
oxalate ion, HC2O4 -, and water? (1 mark) 3) Which of the following is true as a result of the predominant hydrolysis of NaHCO3? (1 mark) 4) Which of the |
|
Hydrolysis: - 911 Metallurgist
spectators are eliminated in net ionic equations (NIE's) for hydrolysis Eg ) Determine whether the salt sodium carbonate (Na2CO3) is acidic, basic or neutral |
|
Project I Acid Base Indicators report
Hydrolysis of NaCl is expected to be negligible, the difference from pH 7 0 insignificant What difference did Net ionic equation for Na2CO3(aq) equilibrium: |
|
HydrolysisLabIntropdf
salts, both anion and cation hydrolyze and the resulting pH depends on which to write a net ionic equation for each hydrolysis sodium carbonate, Na2CO3 |
|
Institute of Mathematics, Physics and Chemistry Department of
The pH scale is based on the molar concentration of the hydronium ion Example 2 (A worked example): write a hydrolysis reaction of Na2CO3 (a salt formed from a α = the molar concentration of dissociated electrolyte / the total molar |
|
Acids, Bases, and Salts Review for Sections 41 - VSB BLOGS
The net ionic equation for the hydrolysis of Na2CO3 is A H2O + Na+ NaOH + H+ B H2O + 2Na+ Na2O + 2H+ C H2O + CO3 2- H2CO3 + O2- D |




















![413 Hydrolysis [eljqo9yz9w41] 413 Hydrolysis [eljqo9yz9w41]](https://i1.rgstatic.net/publication/271749231_Effect_of_sodium_carbonate_concentrations_on_the_degumming_and_regeneration_process_of_silk_fibroin/links/569b051208aeeea985a0e171/largepreview.png)