naming cyclic amides
How do you rename amides?
Now, for primary amides, all you need to do is replace the -ic acid, or -oic acid ending with the suffix “ amide”. All the substituents are numbered by starting from the amide carbon (unless a higher priority group is present) and placed alphabetically just like for naming any other functional group:
What is a cyclic amide called?
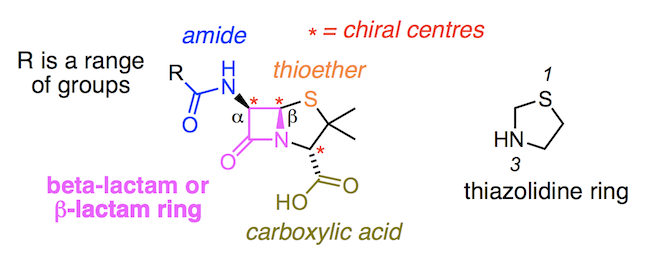
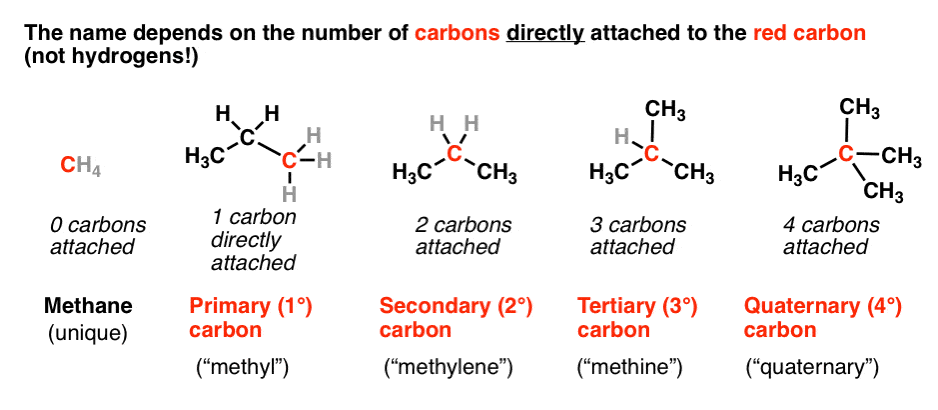
A primary (1°) amide has nitrogen attached to a single carbon; a secondary (2°) amide has the nitrogen attached to two carbons; a tertiary (3°) amide has the nitrogen attached to three carbons. A cyclic amide is called a lactam. When the amide nitrogen has substituents other than hydrogen, we specify them using the prefix N- to avoid confusion.
How are primary amides named?
This action is not available. Primary amides are named by changing the name of the acid by dropping the -oic acid or -ic acid endings and adding -amide. The carbonyl carbon is given the #1 location number.
What is the amide group derived from acetic acid called?
The core −C (=O)− (N) of amides is called the amide group (specifically, carboxamide group ). In the usual nomenclature, one adds the term "amide" to the stem of the parent acid's name. For instance, the amide derived from acetic acid is named acetamide (CH 3 CONH 2 ).
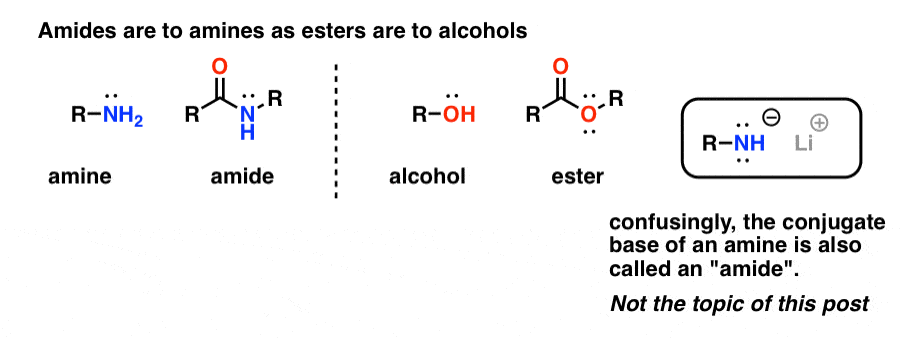
Amides vs Amines: Less Basic, More Acidic
Attaching a carbonyl group to an amine has two drastic effects on the properties of the nitrogen. 1. First, amide nitrogens are considerablyless basic than amine nitrogens. That’s mainly the result of the delocalization of the nitrogen lone pair into the pi bond of the carbonyl.In fact, the most basic position of an amide is not the nitrogen but th
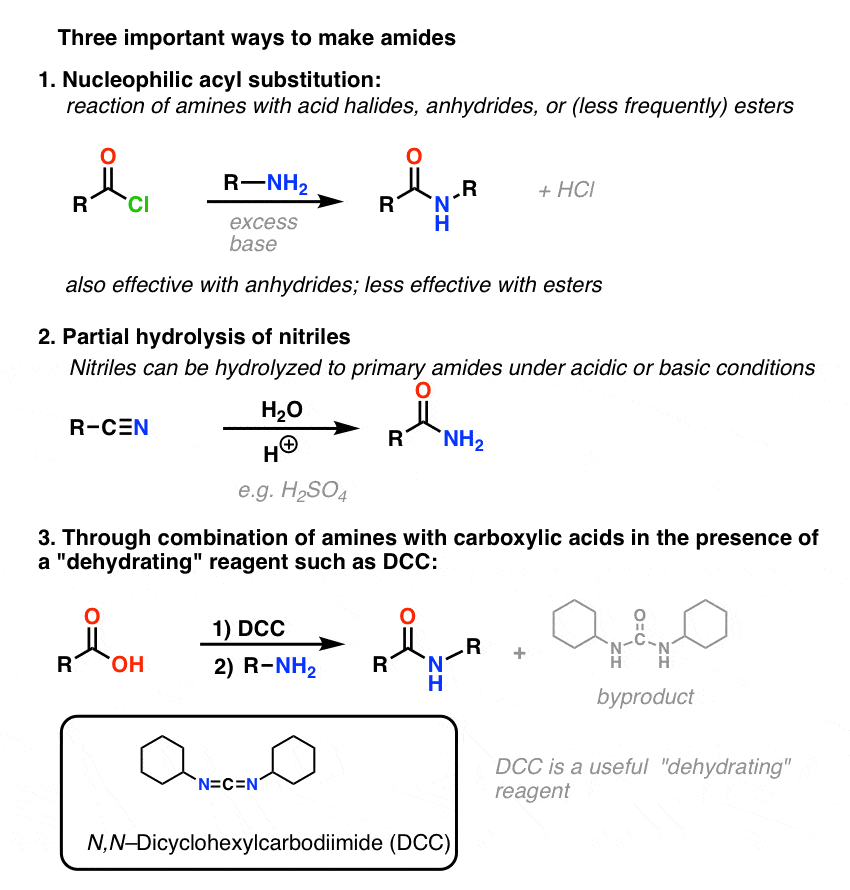
Synthesis of Amides, Part 2: Partial Hydrolysis of Nitriles
One way to think of nitriles is that they are masked carboxylic acids. If treated with aqueous acid and a lot of heat – sledgehammer conditions – they can be hydrolyzed to carboxylic acids. One of the intermediates in this process is a primary amide. So if we use a slightly kinder, gentler sledgehammer technique, sometimes it’s possible to salvage
Synthesis of Amides, Part 3: Use of A Dehydrating Reagent
The synthesis of penicillin V in 1957 by John Sheehan’s group at MIT stands as one of the heroic achievements of postwar-era organic chemistry. The key problem was construction of a cyclic amide (the β-lactam ring) which is extremely unstable under acidic conditions. This was of no small importance, as the β-lactam is also key to penicillin’s mecha
Summary: Three Effective Methods For The Synthesis of Amides
Let’s end by summarizing these three important (but by no means exhaustive) ways to make amides: This concludes our post on the main points of amide nomenclature, properties, and synthesis. For a bonus method of amide synthesis, read on. masterorganicchemistry.com
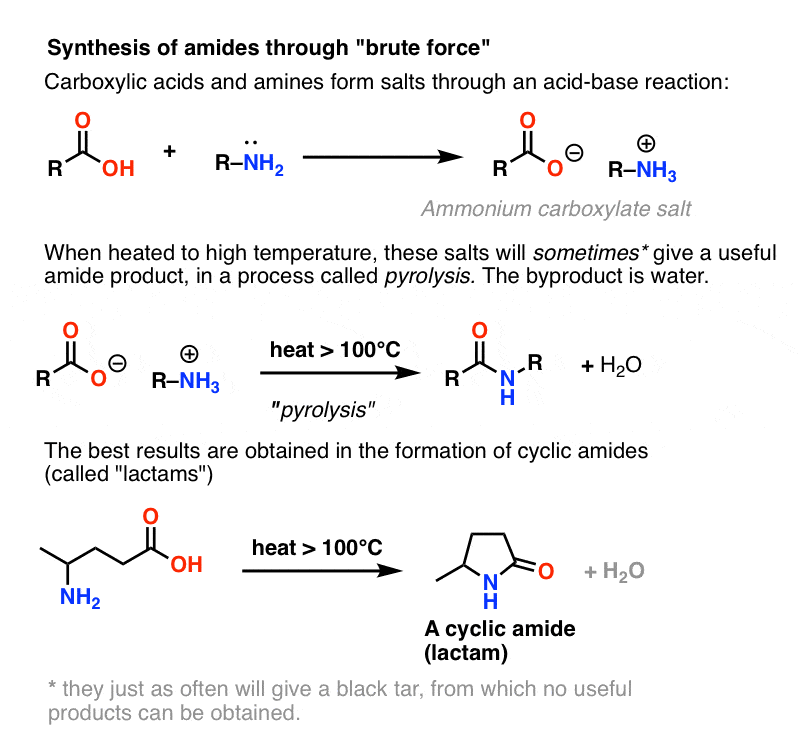
Let Us Briefly Consider A Fourth, Less Important Method: Brute Force
Since it’s usually covered in the textbooks, let’s conclude by considering a fourth possibility – the simplest one imaginable. What if we take a carboxylic acid and combine it with an amine, hoping that an amide will form. What happens? Amines are bases, and carboxylic acids are, well, acids. Add the two together and you get an innocuous salt. Some
Notes
[related articles] A fun, related article: Amides: Humble But Useful(from Chemical & Engineering News). Note 1. There’s also an inductive effect, whereby the electronegative oxygen (electronegativity of 3.44) tugs on the electrons of the attached carbon, which in turn tugs on the electrons of the nitrogen. Note 2. A very common way of carrying out
References and Further Reading
Nitrile hydrolysis: 1. PHENYLACETAMIDE Wilhelm Wenner Org Synth. 1952, 32, 92 DOI: 10.15227/orgsyn.032.0092 The conditions used here for hydration of the nitrile to the amide are rather gentle – this uses a temperature of 40 °C for approximately 1 hr. 2. Halide-directed nitrile hydrolysis James M. Photis Tetrahedron Lett. 1980, 21 (37), 3539-3540 D

Naming Amides

Nomenclature and properties of amides Organic chemistry Khan Academy

IUPAC Nomenclature of Cyclic Amides
|
Chapter 6 Amines and Amides
Learn the IUPAC system for naming amines and amides. carbon groups are connected to the nitrogen atom. ... inhibits cyclic adenosine monophosphate. |
|
Chapter 17: Amines and Amides
Rule 1: Select as the parent carbon chain the longest chain to which the nitrogen atom is attached. Rule 2: Name the parent chain by changing the -e ending of |
|
II. Nomenclature Rules For Alkenes 1. The parent name will be the
The parent name will be the longest carbon chain that contains both carbons of the General Structure. Prefix Name. (if lower priority). Example. -amide. |
|
Naming Carboxylic Acid Derivatives Naming Carboxylic Acid
Amides are named by replacing the –ic acid or –oic acid ending with –amide or by rep- lacing the –carboxylic acid ending with –carboxamide. Cyclic amide are |
|
Short Summary of IUPAC Nomenclature of Organic Compounds
Alkanes are the family of saturated hydrocarbons that is |
|
Root Names for Hydrocarbons
1. the 'root' name indicative of the number of carbon atoms in the longest continuous Amides (contain -CONR): Add “anamide to the root name. |
|
Nomenclature of Organic Chemistry. IUPAC Recommendations and
preferred IUPAC names or names for use in general nomenclature according to the Cyclic amides of amino sulfinic acids and their tautomers are named as ... |
|
Classification and Nomenclature of Amines
Learn the IUPAC system for naming amines and amides. carbon groups are connected to the nitrogen atom. ... inhibits cyclic adenosine monophosphate. |
|
II. CARBOXYLIC ACIDS AND DERIVATIVES One of the more
Cyclic Amides. • Reaction of -NH2 and -COOH on same molecule produces a cyclic amide lactam. • To name |
|
Organic Chemistry
of acid chlorides into carboxylic acids esters |
|
Organic Nomenclature Acids And Derivatives - FacStaff Home Page
Secondly, there is the IUPAC method for naming carboxylic acids carbon δ- Caprolactone Amides Amides are named by dropping the –oic or –ic acid and |
|
Unit One Part 2: naming and functional groups
Ethene - simplest alkene Very important industrially • Carbon is trigonal planar - flat and triangular 10 H H |
|
Esters, Amides, and Related Molecules - Organic Chemistry
15 7 Lactones, Lactams, and Cyclic Anhydrides of acid chlorides into carboxylic acids, esters, amides, or anhydrides (Figure When faced with naming |
|
Chapter 17: Amines and Amides
this chapter amines and amides Amines are Cyclic amines are either secondary or tertiary amines which are designated as Rules for naming amides: CH 3 |
|
Naming CAs Esters and Amides (rules) - Chemistry
The common names of simple carboxylic acids are: ▫ Locate substituents using α, β, γ (letters of the greek alphabet) for the carbon atoms adjacent to the carboxyl |
|
Short Summary of IUPAC Nomenclature of Organic Compounds
IUPAC nomenclature is based on naming a molecule's longest chain of carbons linear or acyclic), or in rings (called cyclic or alicyclic) amides, and nitriles |