nh4cl hydrolysis
What is the hydrolysis reaction for NH4Cl?
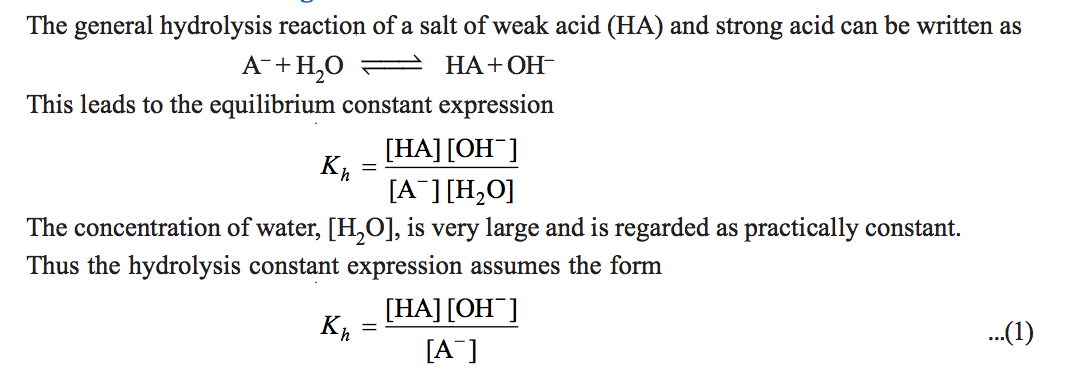
The equation of hydrolysis of ammonium chloride in water is given as; N H 4 C l ( s ) + H 2 O → N H 4 ( a q ) + + C l ( a q ) − , It gets dissociated completely.
Also, it is known that hydronium ion gives hydrogen ion and water on dissociation.What happens when NH4Cl reacts with water?
The salt NH4Cl will first ionise (or dissociates) into the NH4+ and Cl- ions.
The NH4+ ions will lead to the formation of NH4OH and the Cl- will form HCl because of the H3O+ ions from water.
By virtue of HCl being a strong acid and NH4OH being a weak base.Aqueous solution of ammonium chloride (NH4Cl) is :
Acidic due to cationic hydrolysis.

Equation for NH4Cl + H2O (Ammonium chloride + Water)

How to Write the Net Ionic Equation for NH4Cl + NaOH = NaCl + H2O + NH3

How to Balance NH4Cl + NaOH = NH3 + H2O + NaCl (Ammonium chloride + Sodium hydroxide)
|
C:Documents and SettingschmbljMy DocumentspHofsolutions.wpd
To examine the effect of salt hydrolysis on pH For example NH4Cl is formed from the reaction of NH3 |
|
Exp 17 REACTIONS OF SALTS WITH WATER F 08
Hydrolysis as applied to water solutions of inorganic compounds can be defined In a solution of NH4Cl: ... NH4CN will hydrolyze more than either NH4Cl. |
|
Hydrolysis of cupric chloride in aqueous ammoniacal ammonium
[NH3]Total/[Cu]Total. Keywords: Solubility. Cupric diammine chloride. Cupric hydroxychloride. Ammoniacal ammonium chloride solutions. Hidrólisis del cloruro |
|
Clean hydrogen production by the hydrolysis of magnesium-based
5 janv. 2021 purity of the hydrogen produced by the hydrolysis of Mg2Si in NH4F and NH4Cl to highlight the. 25 beneficial effect of F. |
|
Clean hydrogen production by the hydrolysis of magnesium-based
graphite and nickel under Ar was used as the hydrolysis reagent for hydrogen production. The. 12 effects of the solution composition (i.e. NaCl NH4Cl and |
|
An Overview of Ammonium Chloride (NH4Cl) Corrosion in the
Ammonium Chloride (NH4Cl) corrosion is a destructive vapor hydrogen chloride from salt hydrolysis at the distillation unit atmosphere. HCl is gotten. |
|
Enhanced Hydrogen Generation Properties of MgH2-Based
11 mai 2015 The hydrolysis kinetics of MgH2 in different concentrations of ammonium chloride solution. The introduction of a high concentration NH4Cl ... |
|
Regioselective 16-conjugate addition of organocuprate reagents to
aqueous NH4Cl hydrolysis (Table 1 entry 1). In these conditions |
|
MARKING SCHEME
Group A: No Hydrolysis. NaCl Ca(NO3)2 |
|
??????-??????? ???????? ??????????
centration in aqueous solutions on the hydrolysis of magnesium hydride were studied in [6 15]. The presence of even a small amount of NH4Cl (0.5% solution) |
|
C:\Documents and Settings\chmblj\My Documents\pHofsolutionswpd
For example, NH4Cl is formed from the reaction of NH3, a weak base, and HCl, a strong acid The chloride ion will not hydrolyze However, the ammonium ion is the conjugate acid of NH3 and will react with water, producing hydronium ions |
|
Reactions of Salt with Water
Hydrolysis as applied to water solutions of inorganic compounds, can be defined as the reaction of NH4CN will hydrolyze more than either NH4Cl or KCN |
|
Hydrolysis of zinc chloride in aqueous ammoniacal ammonium
When the concentration of ammonium chloride decreases zinc hydrolysis occurs, and zinc hydroxyl compounds are precipitated Therefore, the zinc solubility in |


















![Hydrolysis of Salt and PH of Buffer Solutions - [PDF Document] Hydrolysis of Salt and PH of Buffer Solutions - [PDF Document]](https://0.academia-photos.com/attachment_thumbnails/45776601/mini_magick20180818-28605-1sc8a13.png?1534603464)










![D) (NH4Cl on heating) and (NaNO3 + Zn + NaOH on heating] 80 gm D) (NH4Cl on heating) and (NaNO3 + Zn + NaOH on heating] 80 gm](https://classnotes.org.in/wp-content/uploads/degree-of-hydrolysis-of-SA-and-WB.jpg)