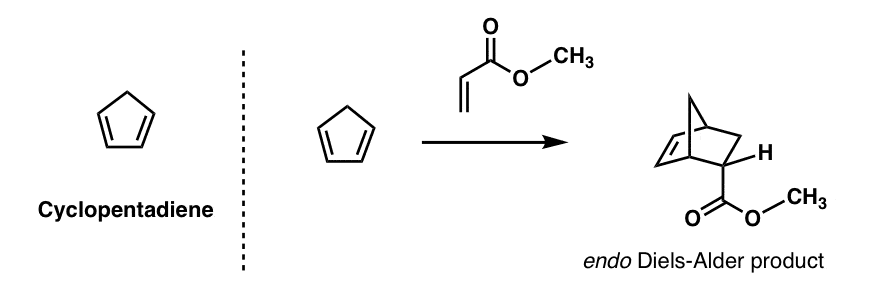
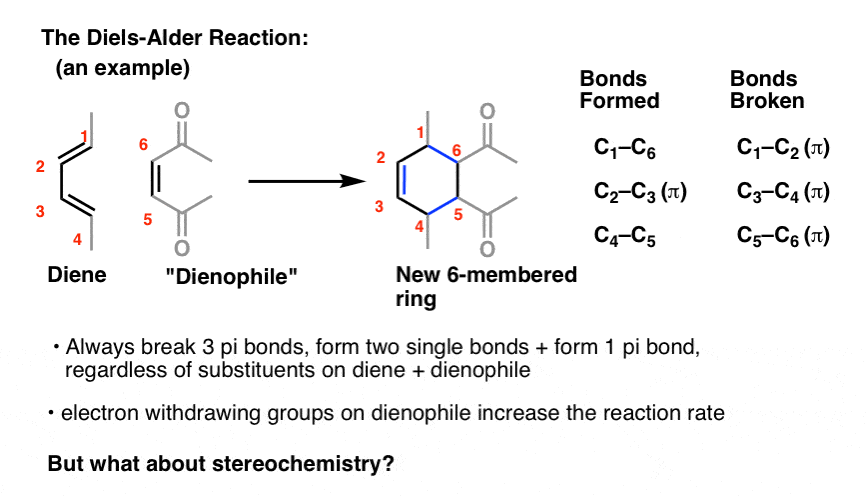
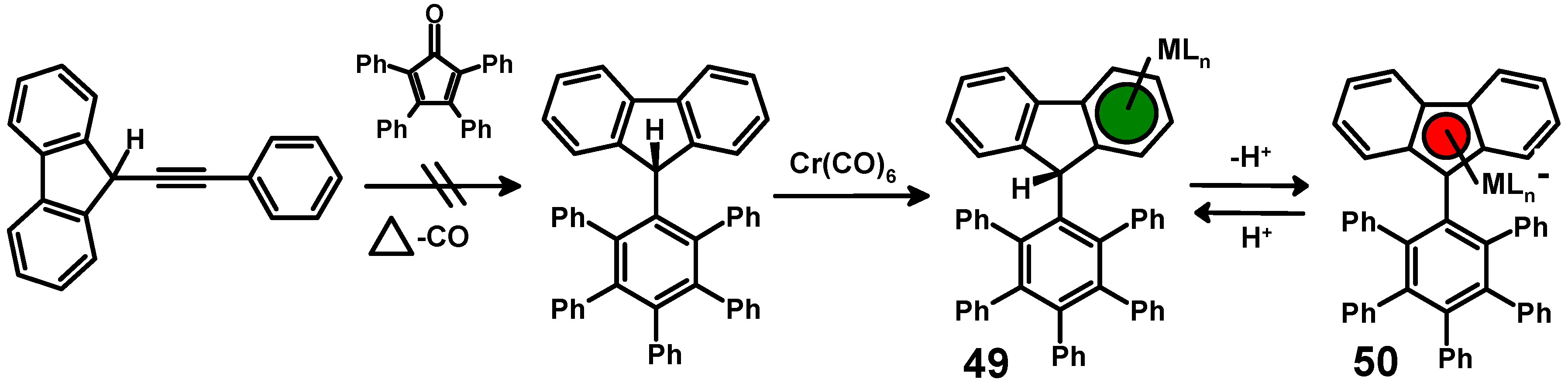
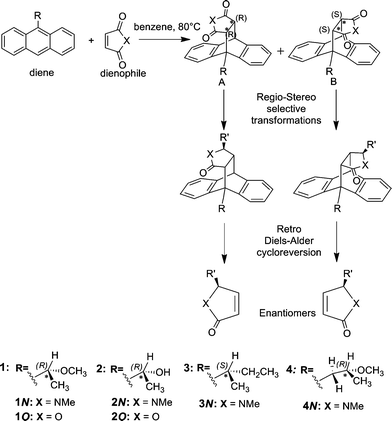
anthracene undergoes diels alder reaction
|
Diels–Alder reactions and electrophilic substitutions with
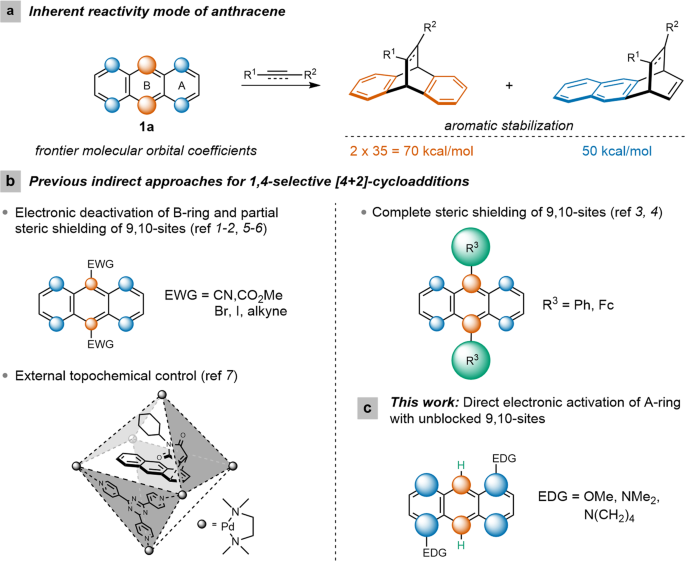
Being an easily available conjugated -electron-rich carbocyclic π system anthracene (1a) has been widely exploited as a classic diene in Diels–Alder reactions wherein its chemical |
What is a thermal Diels Alder reaction of anthracene?
Other thermal Diels–Alder reactions of anthracene with alkenes attached to a heteroatom or halogen include those with tetrachloroethylene, 64 1,3-diacetylimidazolin-2-one, 65 alkenylimmonium salts, 66 vinyl- and propenyl-phosphines and ethyndiylbis (diphenylphosphine oxide) either by heating at reflux in a solvent or by heating in a sealed tube. 67
Is anthracene a classic diene?
Being an easily available conjugated -electron-rich carbocyclic π system, anthracene (1a) has been widely exploited as a classic diene in Diels–Alder reactions wherein its chemical reactivity and transformational effectiveness are subsidized by the partial loss of aromaticity.
How do anthracenes react?
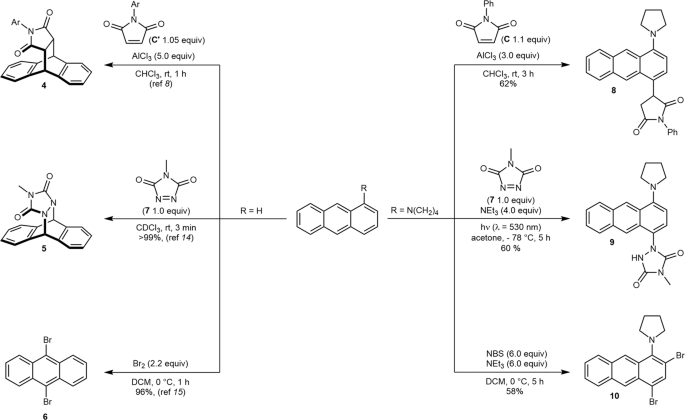
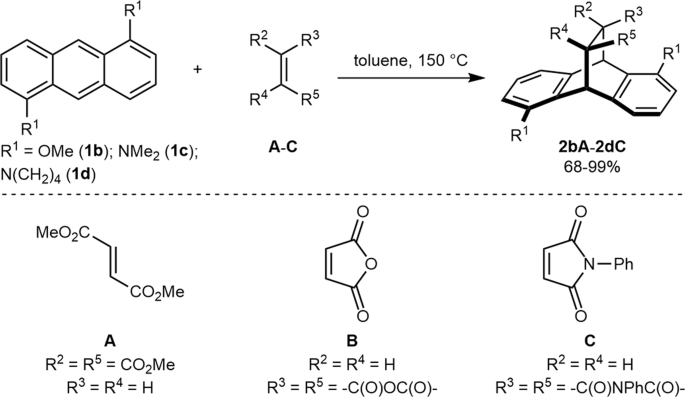
A mechanistic rationale is offered with the aid of detailed computational studies, and finally, synthetic applications are presented. Anthracenes typically undergo Diels-Alder reactions or electrophilic substitutions at the central ring, but can be biased towards reacting at the terminal ring by appropriate blocking groups at the 9,10-positions.
What types of dienophiles are used in Diels Alder reactions?
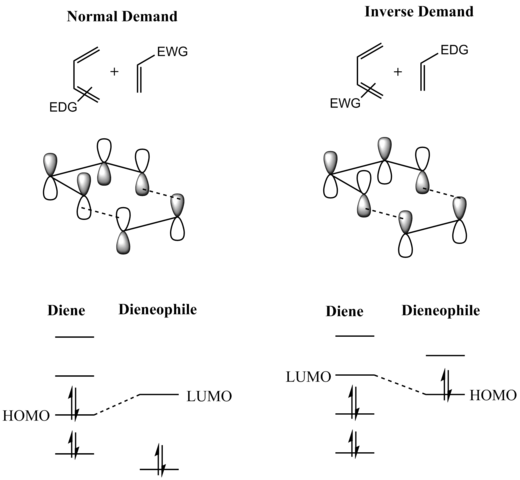
Additions to anthracene The types of dienophile used in the Diels–Alder reactions of anthracene fall broadly into four classes, namely (i) α,β-unsaturated carbonyls, (ii) alkenes attached to a heteroatom or halogen, (iii) alkenes and alkynes and (iv) hetero-dienophiles.
|
Asymmetric [4+2]-cycloaddition of Anthracene Derivatives via
One of the most interesting chemical properties of anthracene is its ability to undergo Diels-Alder reactions with various dienophiles at the 9- and 10 |
|
Asymmetric [4+2]-cycloaddition of Anthracene Derivatives via
One of the most interesting chemical properties of anthracene is its ability to undergo Diels-Alder reactions with various dienophiles at the 9- and 10 |
|
The Diels-Alder Reaction of Anthracene with Nitroölefins. A New
Anthracene is known to undergo Diels-Alder reactions with many dienophiles2 but until recently there were no published reports of a reaction be- tween |
|
Synthesis and transformations of a few 9-(pent-4-yn-1-yl)anthracene
9-(Pent-4-yn-1-yl)anthracene-type compounds can potentially undergo intramolecular Diels-Alder (IMDA) reaction to form 911-annulated dibenzobarrelenes |
|
Enantioselective Diels–Alder reaction of anthracene by chiral
14 июн. 2019 г. strategy the carbocation precursor can undergo facile ionic dissociation upon mild external stimulation such as polar sub- strates (such as ... |
|
BP301(T) Pharmaceutical organic chemistry-II Unit-iv
undergoes addition and electrophilic substitution reactions These reactions ... Anthracene undergoes Diels-Alder reaction at 9 10 positions and form endo ... |
|
Diels-Alder Reactions of trans-44
https://pubs.acs.org/doi/pdf/10.1021/ja01595a035?rand=o2dg51r8 |
|
Synthesis Characterization
https://repository.lsu.edu/cgi/viewcontent.cgi?article=6922&context=gradschool_disstheses |
|
Synthesis of Extended Linear Aromatics Using Tandem Diels-Alder
Anthracene also undergoes photochemical 4 +. 4 cycloaddition reactions across the 910 positions toform a thermoreversible dimer. This reaction of anthracene. |
|
Organic & Biomolecular Chemistry
19 мая 2020 г. show the anthracene transfer to take place in a synchronous retro Diels–Alder/Diels–Alder reaction: an anthracene molecule dissociates from ... |
|
Diels-Alder reactivity of polycyclic aromatic hydrocarbons. 1. Acenes
holds for tetrabenzanthracene. Attempts to observe the Diels-Alder reaction of naphthalene phenanthrene |
|
Simple and Efficient Purification Protocol of 9-(Trifluoroacetyl
anthracene the major impurity associated in its synthesis. The protocol utilizes a chemose- lective thermal Diels–Alder reaction |
|
BP301(T) Pharmaceutical organic chemistry-II Unit-iv
Chemical:-The reactions of naphthalene are essentially the same as those of Anthracene undergoes Diels-Alder reaction at 9 10 positions and form endo ... |
|
Synthesis and transformations of a few 9-(pent-4-yn-1-yl)anthracene
9-(Pent-4-yn-1-yl)anthracene-type compounds can potentially undergo intramolecular Diels-Alder (IMDA) reaction to form 911-annulated dibenzobarrelenes. |
|
Chem365 Labbook-2011
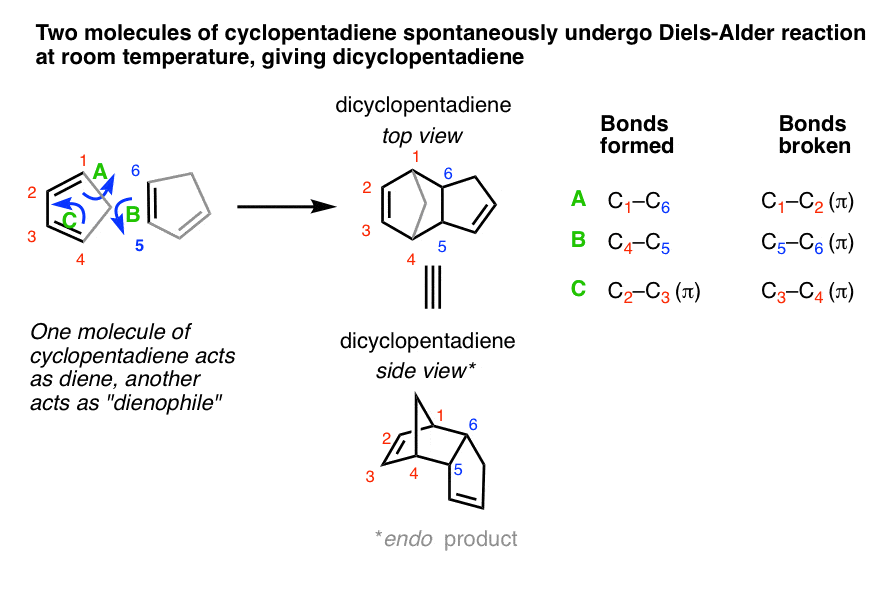
General Diels-Alder Reaction: A conjugated “diene” reacts with a “dienophile” Reaction: Anthracene (1) will serve as a student-friendly low-smell Diels-. |
|
Pericyclic Reactions and Organic Photochemistry S. Sankararaman
In other words Anthracene undergoes Diels-Alder reaction |
|
Pathway-Dependent Post-assembly Modification of an Anthracene
08-Aug-2016 Here we show how a new anthracene-edged FeII. 4L6 cage 1 undergoes PAM via Diels?Alder reaction with tetracyano-. |
|
Diels-Alder Reaction
Overview of Actual Reaction: Anthracene (1) will serve as a student-friendly low-smell Diels-. Alder diene with the labeled carbons functioning as the |
|
Photocrosslinking Polymeric Ionic Liquids via Anthracene
16-Jul-2018 external stimulus undergoes changes in mechanical properties without ... a [4? + 2?] Diels-Alder reaction with anthracene |
|
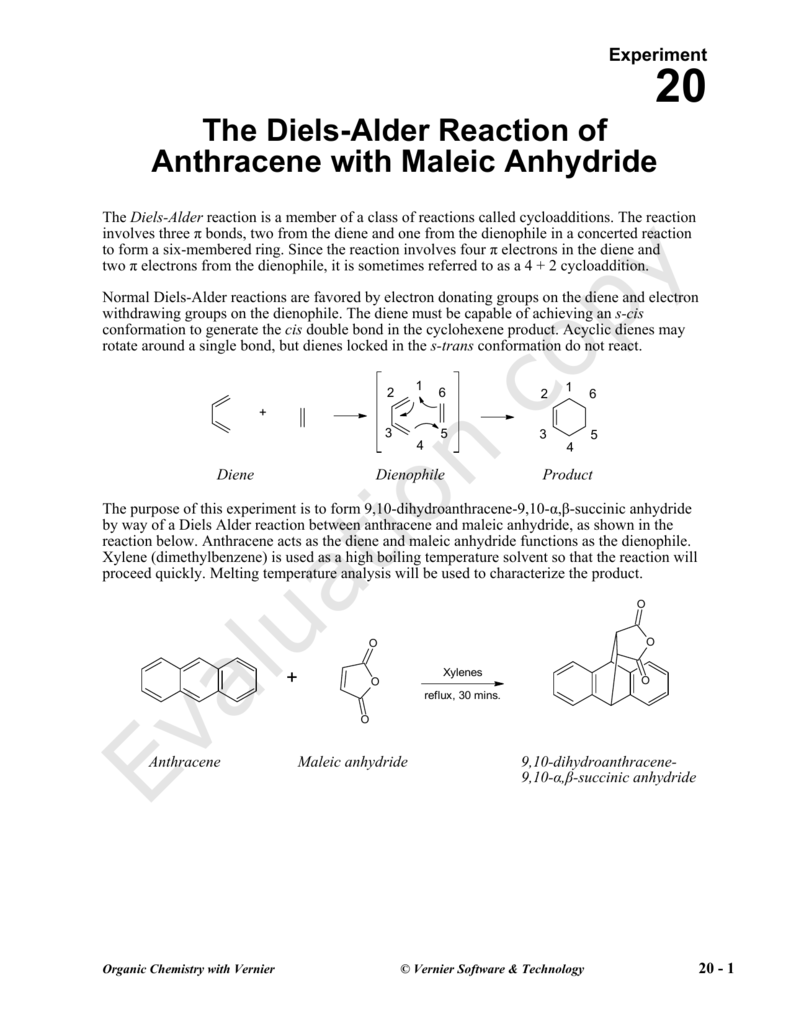
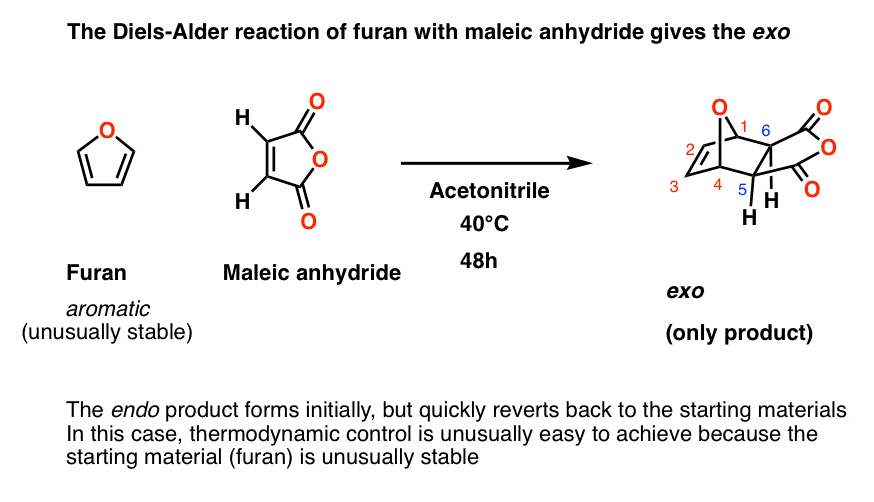
The Diels-Alder Reaction of Anthracene with Maleic Anhydride
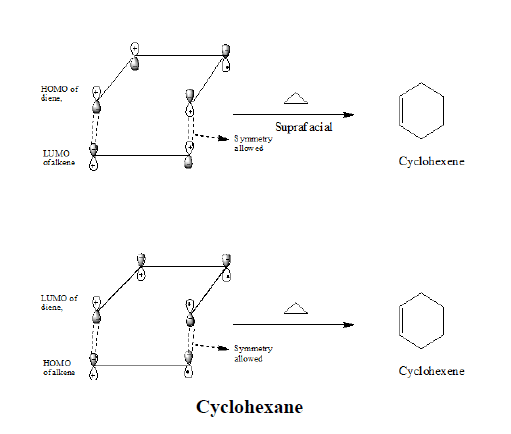
The Diels-Alder reaction is a member of a class of reactions called cycloadditions The reaction involves three π bonds, two from the diene and one from the dienophile in a concerted reaction to form a six-membered ring Anthracene acts as the diene and maleic anhydride functions as the dienophile |
|
Diels-Alder reaction between naphthalene and N-phenylmaleimide
12 The rate of the Diels-Alder reaction between substituted anthracenes and activated dienophile (7) increases by 5•104 times 13 It follows from these data that the |
|
Stereoelectronic effects in the Stereoselectivity of the Diels-Alder
The characteristic feature of anthracene behaving as a diene and its ability to undergo Diels-Alder reaction with various dienophiles have been well documented |
|
DIELS-ALDER REACTION
we have chosen to include related aspects of the Diels-Alder reaction whenever they Substituted butadienes also undergo addition with maleic anhydride Isoprene product with a partially hydrogenated phenanthrene nucleus 33 |
|
Experiment 20 — Pericyclic reactions
Pre-lab preparation (1) Read text section 22 6 (2) Write the potential Diels-Alder reactions of maleic anhydride and anthracene, and (3) estimate the reaction |
|
Abdel Al-Saeedipmd - Oriental Journal of Chemistry
of the Diels-Alder reaction of 9-substituted anthracene with a variety of dienophiles Key words: the ability to undergo cycloaddition reactions with a variety of |
|
High stereoselectivity in the Diels-Alder reaction of - NOPR
1-Succinimidoanthracene undergoes Diels-Alder reaction with maleic anhydride to give mainly the anti anthracene 1 with the unsymmetrical dienophile 2 |