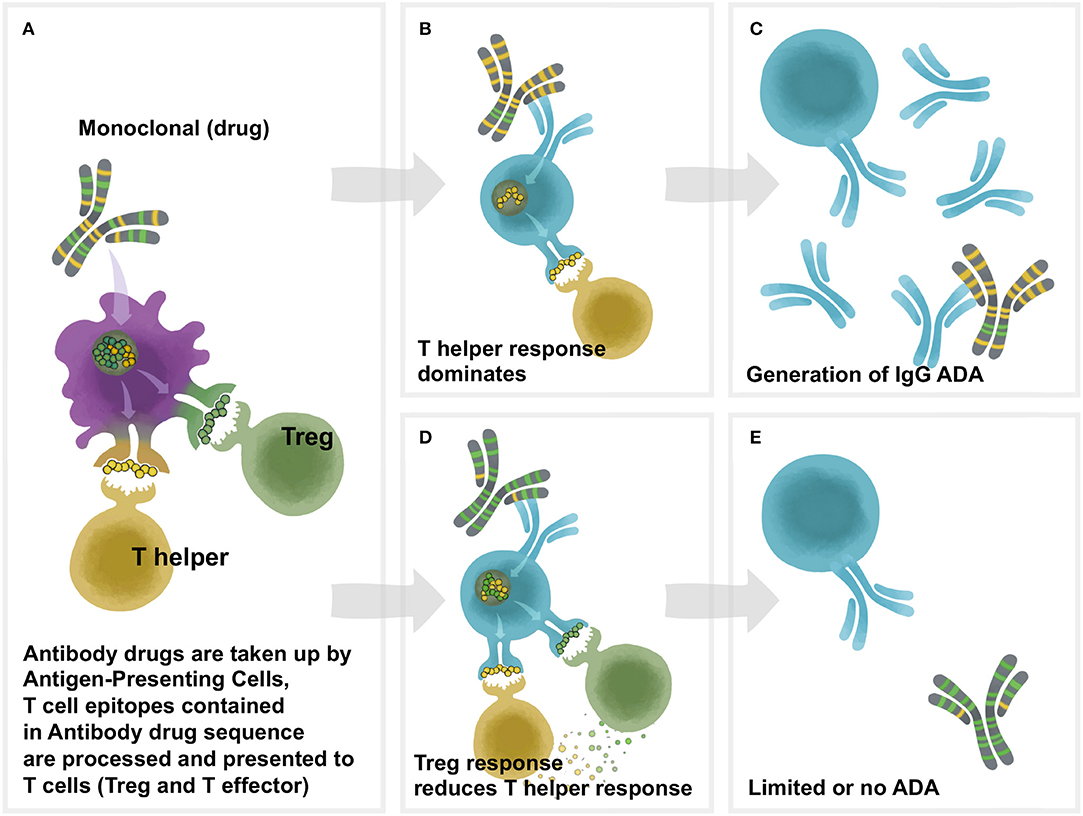
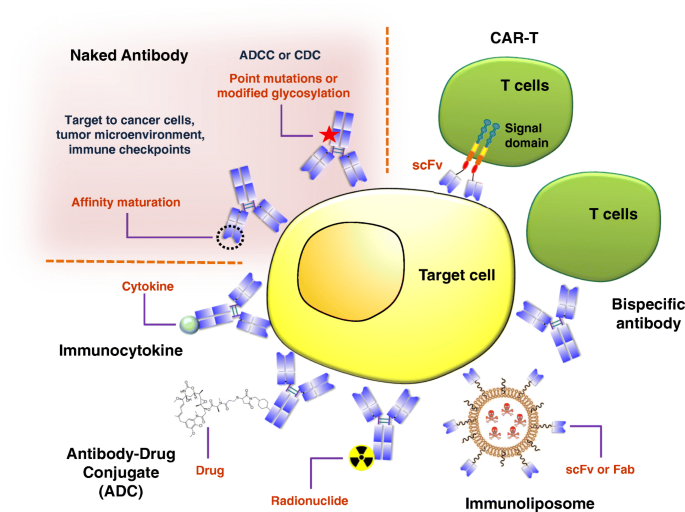
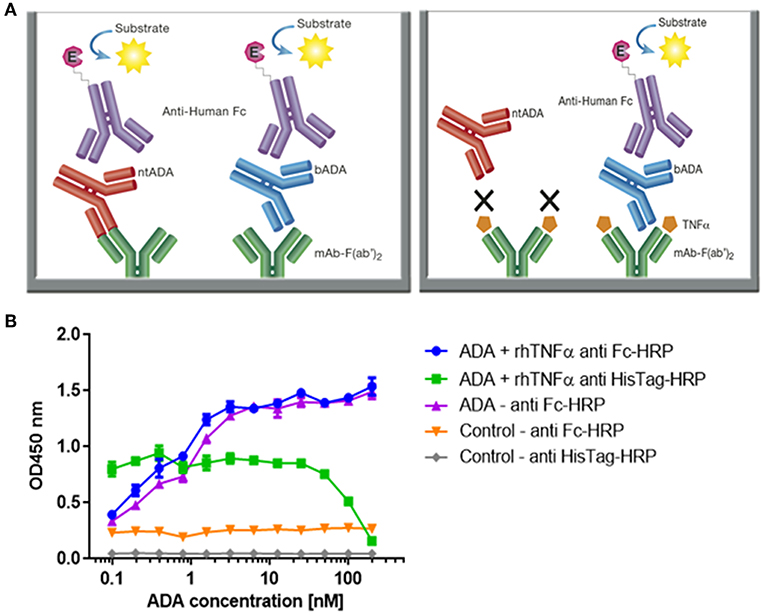
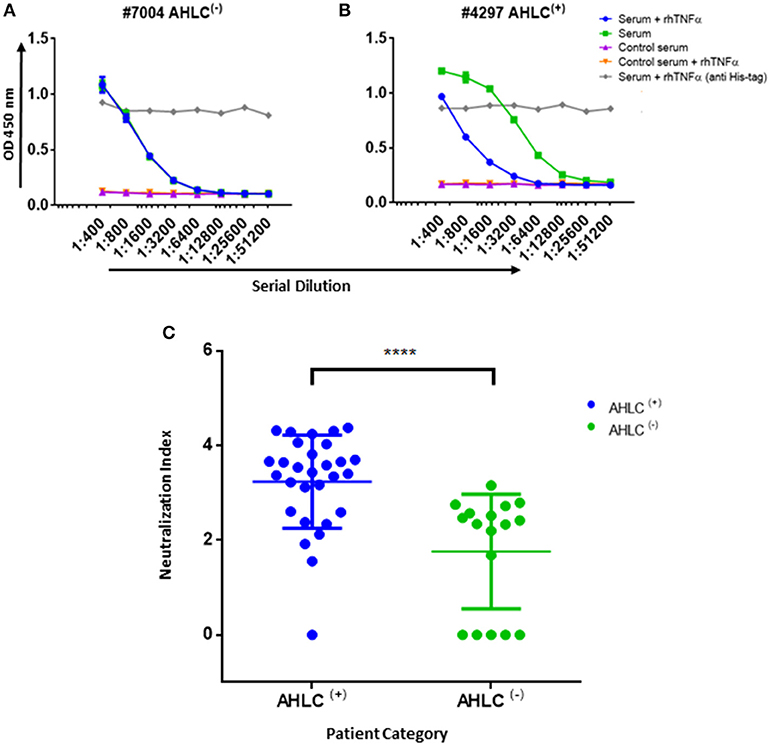
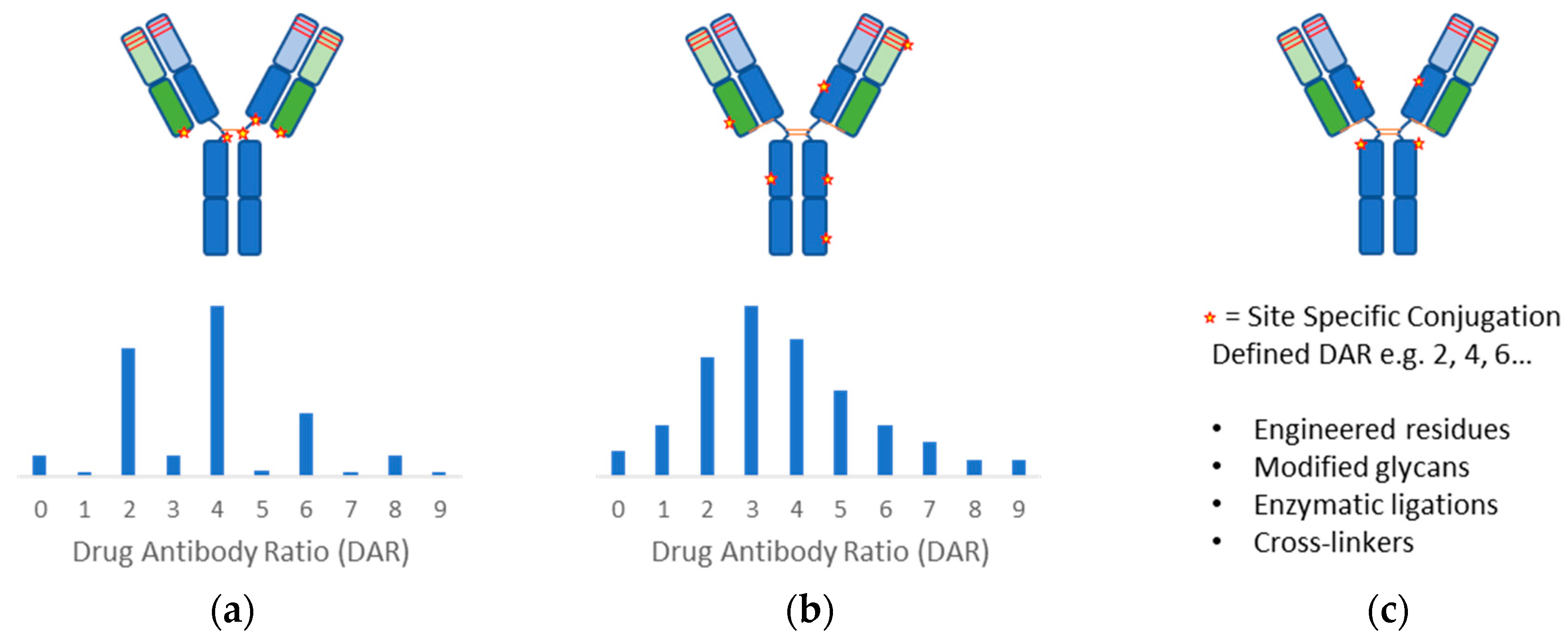
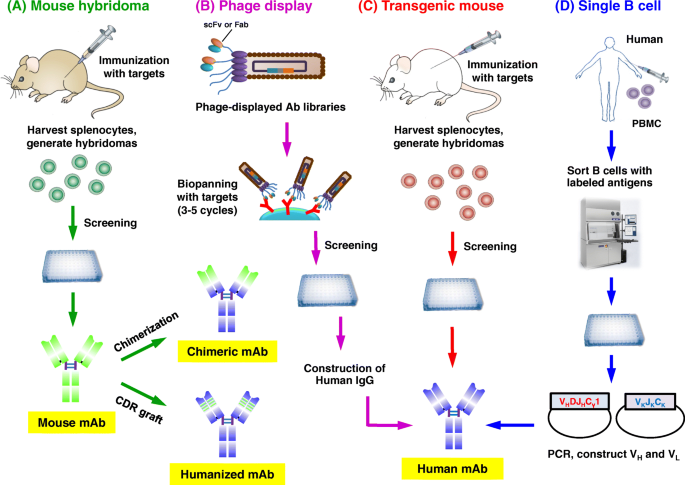
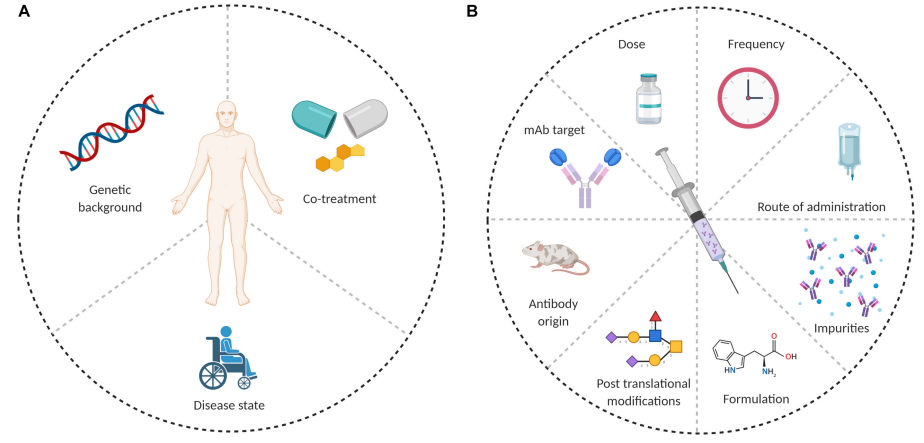
anti drug antibodies definition
|
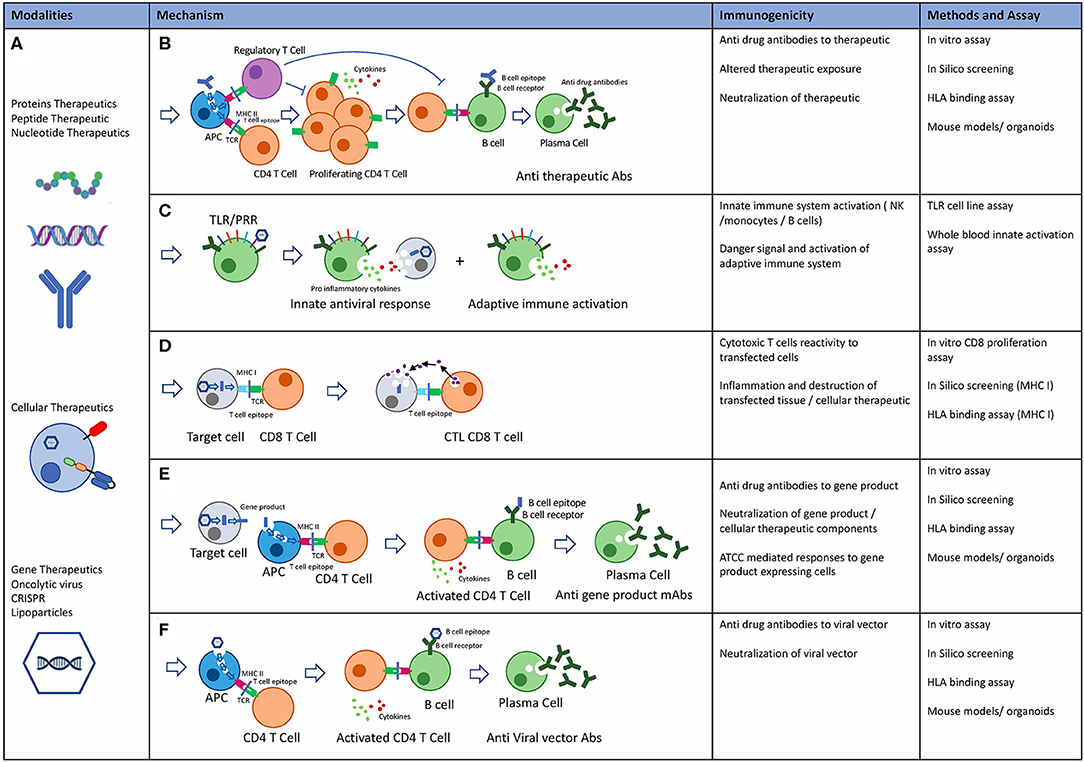
The immunogenicity of therapeutic proteins
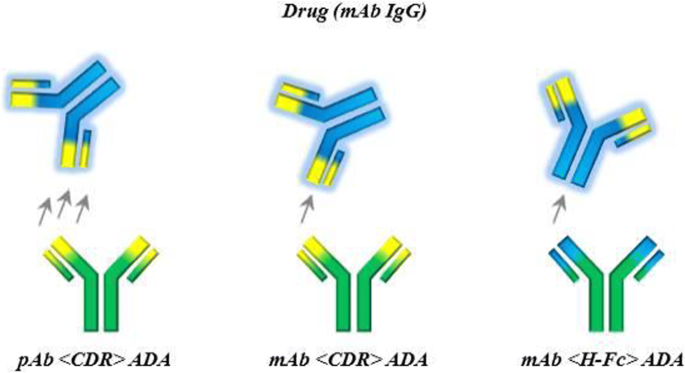
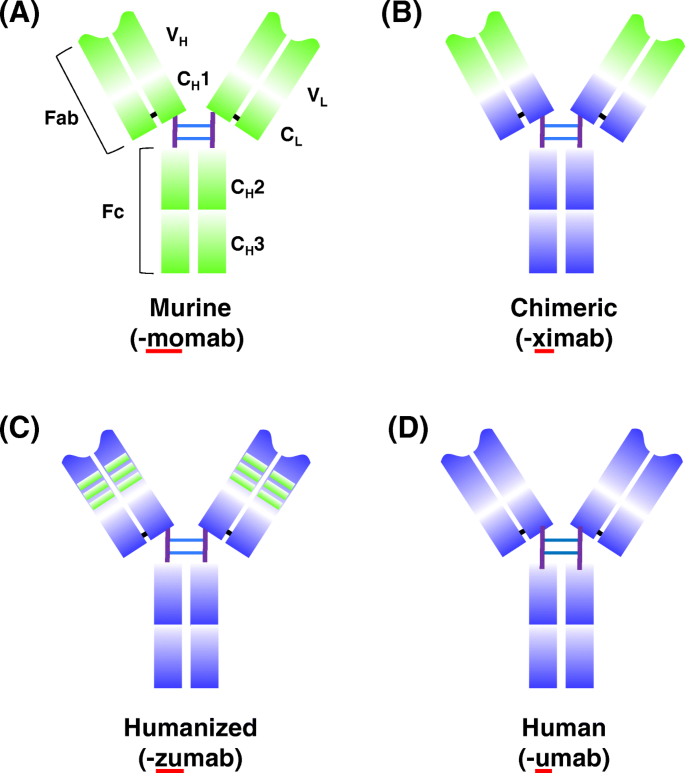
Antibodies directed against TP (anti-drug antibodies ADA) may consist of IgM IgG IgE and/or IgA isotypes interactions between antigen presenting cells T-helper cells B-cells and |
|
Guideline on Immunogenicity assessment of therapeutic proteins
Immunogenicity therapeutic proteins anti-drug antibodies (ADA) assays assay strategy binding antibodies neutralising antibodies risk factors safety efficacy pharmacokinetics risk management integrated summary of immunogenicity |
Should anti-drug antibodies be measured in animal studies?
Measurement of anti-drug antibodies in animal studies may be needed as part of repeated dose toxicity studies, in order to aid in the interpretation of these studies (as discussed in “ICH S6 (R1) Harmonized Tripartite Guideline on preclinical safety evaluation of biotechnology-derived pharmaceuticals.”).
Should anti-drug antibody response be compared to a reference product?
In the development of similar biological medicinal products (biosimilars), the comparison of the anti-drug antibody response to the biosimilar and the reference product in an animal model is not recommended as part of the biosimilar comparability exercise, due to the low predictivity for the immunogenicity potential in humans.
How do anti-drug antibodies affect pharmacokinetic properties?
The resulting anti-drug antibodies modify the pharmacoki-netic and pharmacodynamic properties of the drug and, byblocking the drug-target interac-tion, reduce the effects of the treatment. Anti-drug antibodies may also increase the risk of hypersensitivity reactions by the formation of immune complexes.
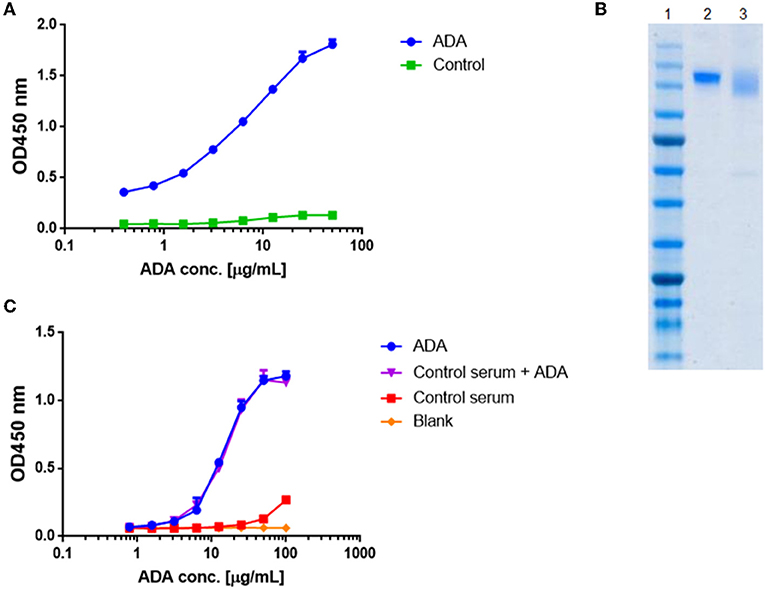
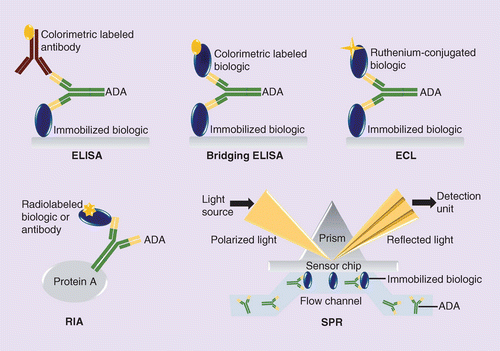
What are the different types of antidrug antibodies (ADA) detection immunoassay formats?
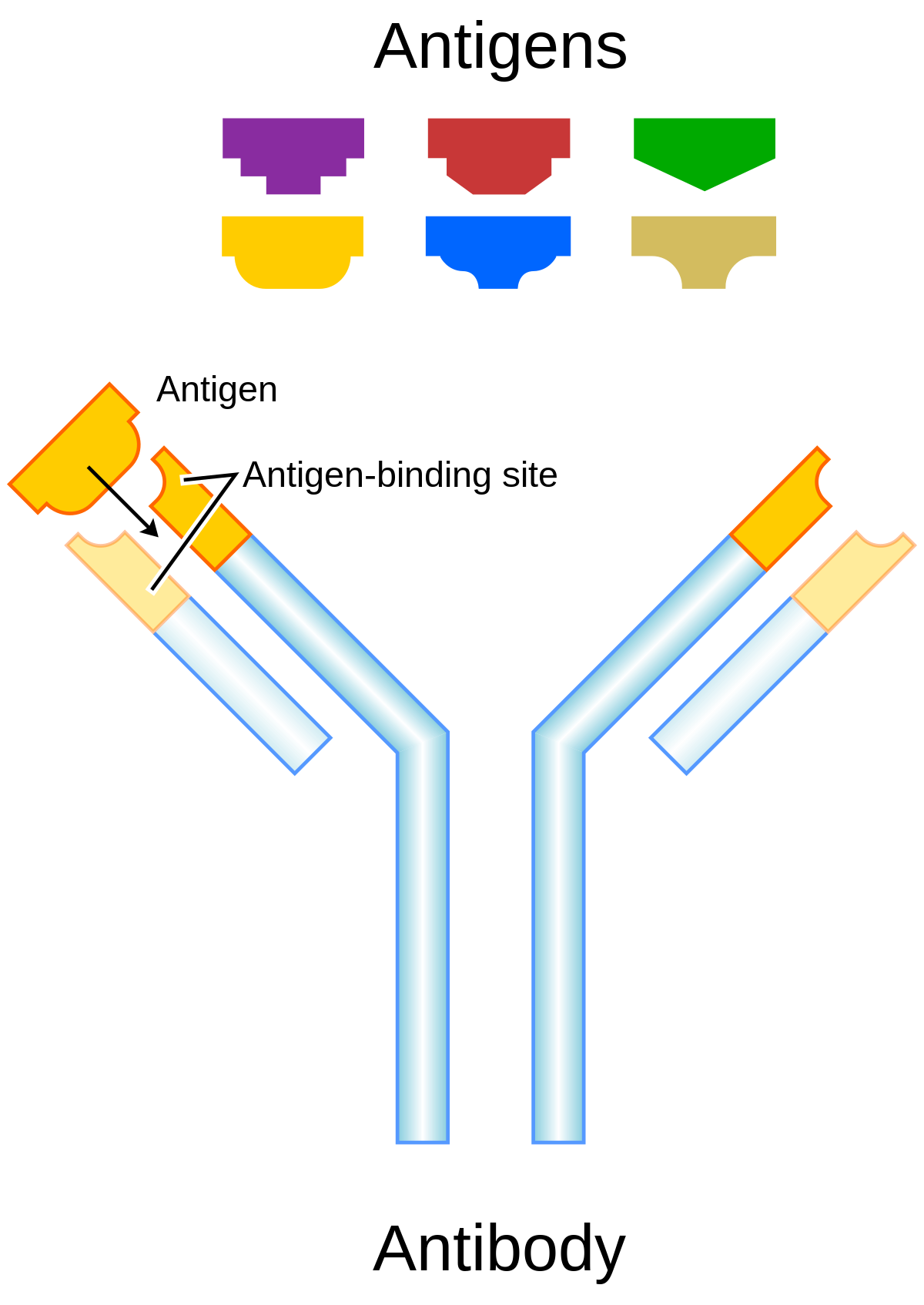
Anti‐drug antibodies (ADA) detection immunoassay formats. (a,b) Direct immunoassay methods. Drug ‘D’ is (a) coated on the assay plate directly or (b) captured on a streptavidin‐coated plate via a biotin linker (orange triangle). Test sample is added and ADA (Y‐shaped red symbol) are captured by platebound drug.
|
Immunogenicity Testing of Therapeutic Protein Products
Validation of Immunoassays to Detect Anti-Drug Antibodies and 1106.1 Immunogenicity Assays — Design and defining the cut-point and are discussed in detail in ... |
|
EBF recommendation for stability testing of anti-drug antibodies
ADA: Anti-drug antibody; AVA: Anti-vaccine antibody. Box 1. Definition of short- and long-term stability as per the US FDA guidelines. Short-term stability. |
|
Immunogenicity Information in Human Prescription Therapeutic
Products — Developing and Validating Assays for Anti-Drug Antibody Detection (January 2019) and other applicable FDA guidances. 10 See the guidance for industry |
|
Proposed 1st WHO International Reference Panel for Infliximab anti
23 сент. 2022 г. A panel of two human monoclonal antibodies against human infliximab with defined ... therapeutic antibodies and anti-drug antibodies. Nature 9: ... |
|
Guideline on Immunogenicity assessment of therapeutic proteins
18 мая 2017 г. Immunogenicity therapeutic proteins |
|
STATISTICAL ANALYSIS PLAN VERSION: FINAL
1 мар. 2015 г. Drug Dictionary (WHODD). Subjects will be counted once in all ATC ... anti-REGN2222 antibodies on drug exposure. The impact of treatment ... |
|
Immunogenicity Assessment for Therapeutic Protein Products
Furthermore the presence of anti-drug antibody (ADA) alone is not necessarily predictive of anaphylaxis or other hypersensitivity reactions. Correlation |
|
Immunogenicity Information in Labeling 04052022
5 апр. 2022 г. Immunogenicity: Unknown Clinical Effects of Anti-Drug Antibodies ... 1 “Narrow Therapeutic Index” drugs are defined for the purposes of this ... |
|
Eylea INN-aflibercept
22 янв. 2015 г. Anti-drug antibodies. ADR. Adverse drug reaction. AE. Adverse event. AMD ... considered limited as the definition of retinal ischemia as number of ... |
|
Letter: predicting treatment discontinuation in inflammatory bowel
demonstrated immunogenic failure (defined by undetectable anti- TNF trough levels and high levels of anti- drug antibodies using drug sensi- tive assay) |
|
Immunogenicity Testing of Therapeutic Protein Products
purposes of this guidance immunogenicity is defined as the propensity of a Validation of Assays to Detect Anti-Drug Neutralizing Antibody for a broader ... |
|
Guideline on Immunogenicity assessment of therapeutic proteins
18 mai 2017 Immunogenicity therapeutic proteins |
|
EBF recommendation for stability testing of anti-drug antibodies
ADA: Anti-drug antibody; AVA: Anti-vaccine antibody. Box 1. Definition of short- and long-term stability as per the US FDA guidelines. Short-term |
|
STATISTICAL ANALYSIS PLAN VERSION: FINAL
Analysis Plan (SAP) and agreed on the planned analysis defined in this document for Analysis of Pharmacokinetics and Anti-Drug Antibody Data . |
|
Immunogenicity Assessment for Therapeutic Protein Products
For the purposes of this guidance immunogenicity is defined as the Furthermore |
|
Development of anti-drug antibodies against adalimumab and
associations between the development of antidrug antibodies and diminished serum Remission was defined as a DAS28 of less than 2.6 at all consecutive. |
|
Letter: predicting treatment discontinuation in inflammatory bowel
bowel disease— anti- TNF trough levels and anti- drug antibodies. Authors' reply in the French cohort) the definition of drug discontinuation and the. |
|
Guideline on immunogenicity assessment of biotechnology-derived
Characterisation of antibodies to a therapeutic protein . comparison of the incidence of anti-drug antibodies. Deviations from this concept should be ... |
|
LIBTAYO INN -cemiplimab
20 mai 2021 Assessment report. EMA/319415/2021. Page 3/118. List of abbreviations. Abbreviation. Definition. ADA. Anti-drug antibody. |
|
Anti-Drug Antibodies: Emerging Approaches to Predict Reduce or
31 mai 2018 Keywords: anti-drug antibodies; biotherapeutics; immunogenicity; ... “minimal epitope” defined as the residues contributing the most to ... |
|
Anti-Drug Antibody Testing In Toxicity Studies
Outline • The immune response to biological drugs • Types and examples of anti -drug antibodies • Evaluation of anti-drug antibodies in toxicity studies |
|
Guideline on Immunogenicity assessment of therapeutic proteins
18 mai 2017 · Keywords Immunogenicity, therapeutic proteins, anti-drug antibodies (ADA), of risk-based approach to immunogenicity which means that the |
|
Programming Support for Anti-Drug Antibody - PharmaSUG
anti-drug antibodies (ADA) generated in unwanted immune response may sustain, clear, Definition: Positive if ADASMPL = Positive ; Negative if ADASMPL in |
|
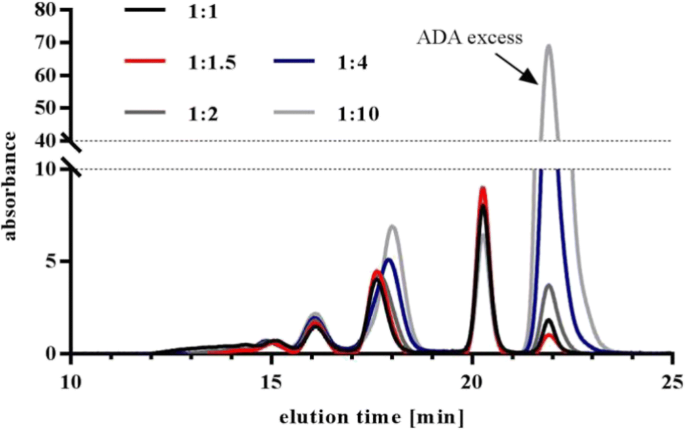
Strategies to Improve Free Drug Tolerance in Anti-Drug - Celerion
increasing, drug tolerance in anti-drug antibody (ADA) assays is of growing concern Drug tolerance is generally defined as the maximal amount of free drug in |