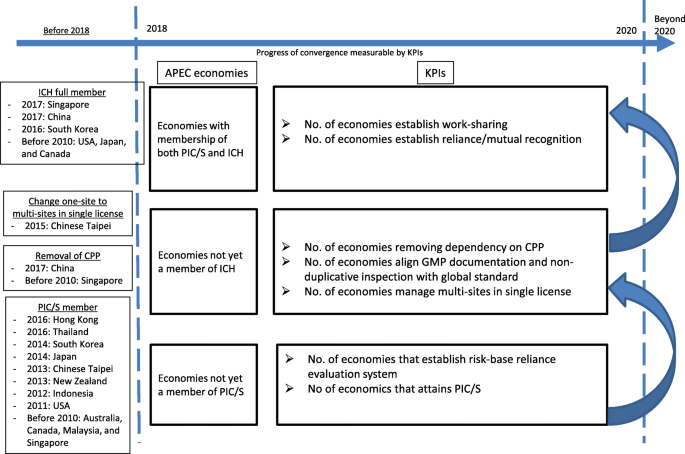
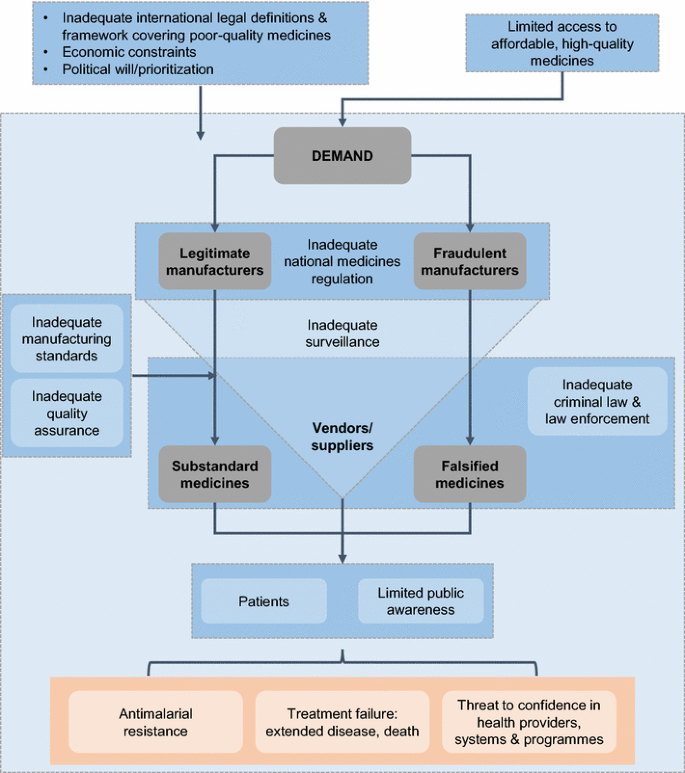
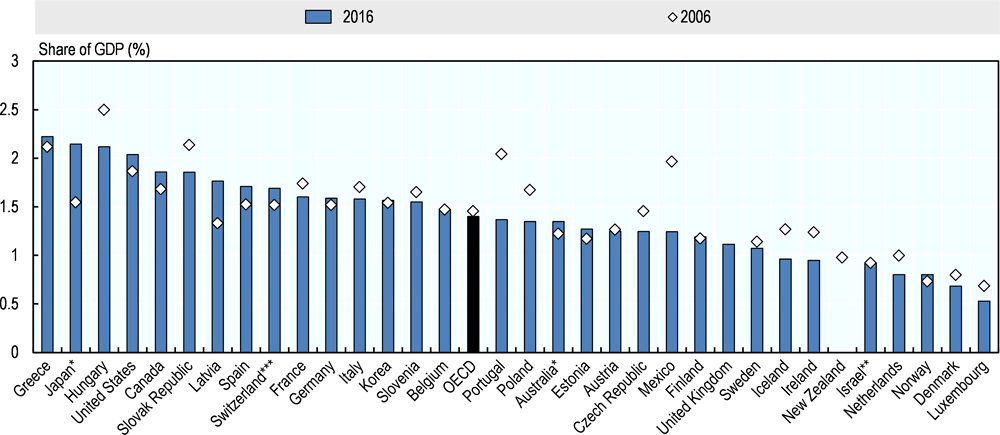
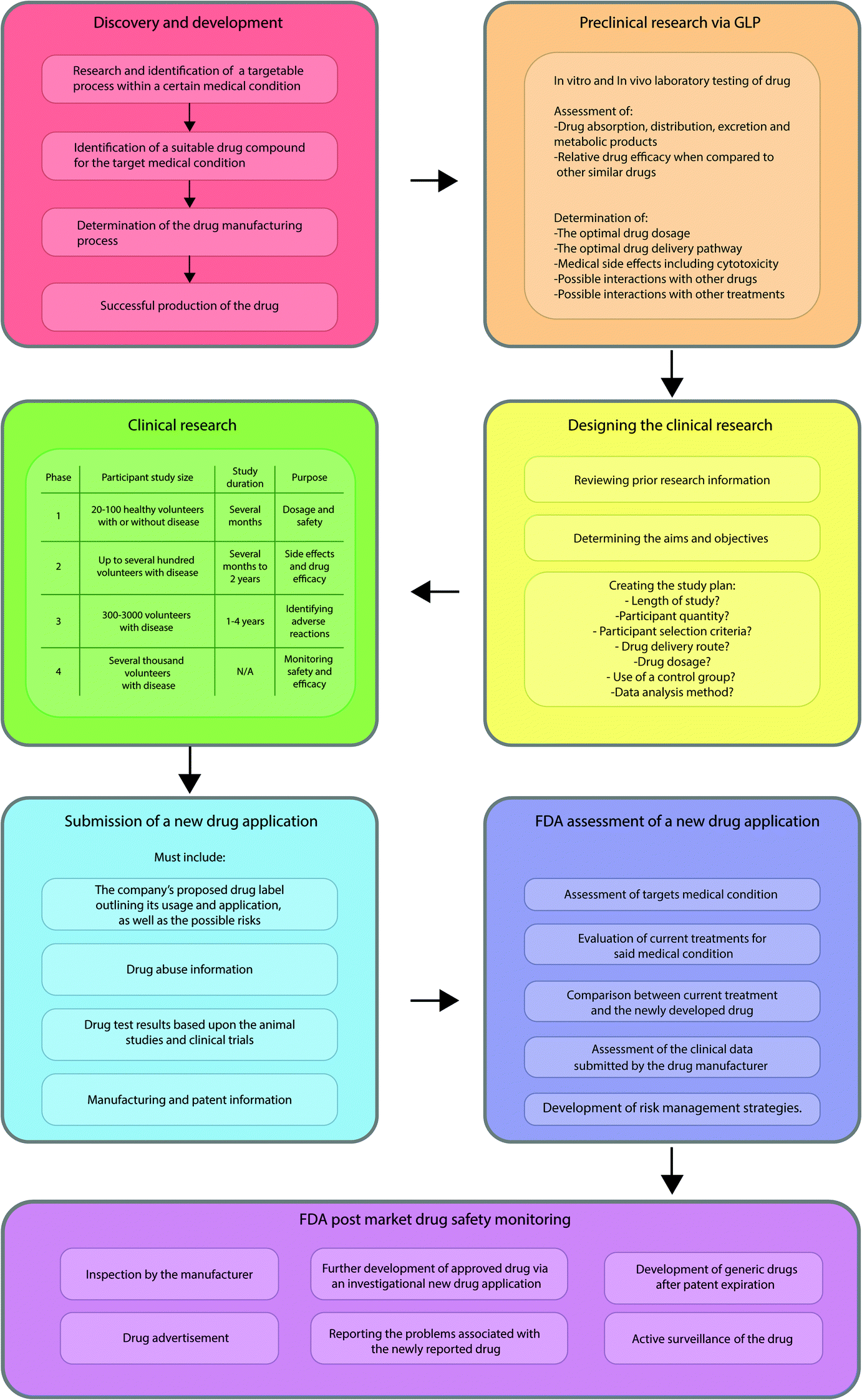
International Regulators Consortium International Generic Drug
|
Regulatory harmonization - WHO World Health Organization
The pre-market review of generic medicines puts mounting pressures on health regulatory group of regulators to launch the International Generic Drug Regulators Pilot (IGDRP), aimed at marketing applications to a consortium of RAs |
|
Towards global knowledge of the international medicine regulatory
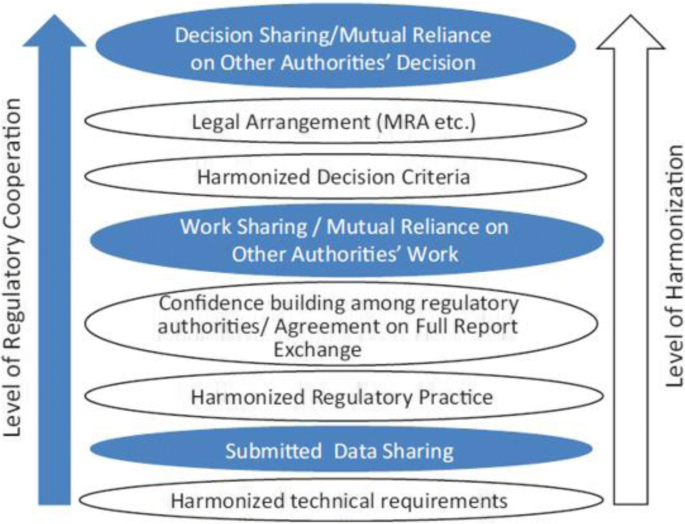
IGDRP—International Generic Drug Regulators Pilot IPRF—International Pharmaceutical Regulators Forum IRC—International Regulators Consortium |
|
ICMRA - Mapping of generic initiatives - European Medicines Agency
International Generic Drug Regulators' Pilot Quadrilateral Consortium Generics regulatory authorities in some EU Member States in support of marketing |
|
ROADMAP TO 2020 - FDAnews
7 oct 2016 · The official launch of the International Generic Drug Regulators Pilot shared interest and commitment by a consortium of RAs to explore |
|
International Regulatory Co-operation and the Use of Foreign
IGDRP = International Generic Drug Regulators Pilot (multilateral) HoA Consortium = Heads of Agency Consortium (quadrilateral) Infosharing, Staff exchange |
|
ACSS - Health Sciences Authority
and Switzerland (ACSS) Consortium - Generic Medicines Work Sharing Trial ( GMWST) Regulatory authorities face very similar challenges, such as increasing workload and The purpose of this international information sharing and |
|
Global Comparator Product for Biosimilar Development and Waiving
4 nov 2019 · Next steps in the biosimilars regulatory framework: global biosimilar Founded in March 1997 as the International Generic Pharmaceutical Alliance ACSS - Australia, Canada, Singapore, Switzerland Consortium – |
|
Global Comparator Product for Biosimilar Development and Waiving
21 mar 2019 · Kox, Secretary General IGBA International Generic and Biosimilar medicines Association to respond to the increasingly global face of drug development ACSS (Australia, Canada, Switzerland and Singapore) Consortium reduce regulatory barriers for applicants seeking to register generics, while |
|
The ACSS Consortium - Sindusfarma
29 jui 2017 · the International Generic Drug Regulators Programme (IGDRP); ○ Demonstrate a model for other collaborations among regulatory authorities |
|
Better Regulation of Medicines Means Stronger Regional Health
The three common pillars of medicines regulation are quality, International Consortium Aims to Facilitate Availability of Generic Drugs for Patients Through |